
Somatostatin receptor imaging in nasopharyngeal cancer
Introduction
Somatostatin is a neuropeptide better known for its role in inhibiting the secretion of growth hormone, insulin and glucagon. Somatostatin analogues, octreotide, lanreotide and pasireotide are currently being used in the management of acromegaly and neuroendocrine tumors. Somatostatin exerts its function by binding to its receptors. There are five subtypes of somatostatin receptors (SSTRs, sstr1–5) with varying tissue distribution and function. They have been reported to be expressed in the brain, pituitary gland, pancreas, gastrointestinal tract, adrenal glands, immune cells and several human tumors (1-3).
Somatostatin receptors in NPC
Neuroendocrine tumors in particular express high levels of somatostatin receptor subtype 2. Radiolabeled octreotide scans (Octreoscan) identify sites of neuroendocrine tumor involvement and select for patients who may be suitable for peptide receptor radionuclide therapy (PRRT).
It is thus very exciting that somatostatin receptors have been reported in nasopharyngeal cancer (NPC). The first report of somatostatin receptors in NPC was by Loh in 2002. Using receptor autoradiography, Loh et al. found nine out of 12 NPC histology specimens expressed moderate to high levels of somatostatin receptor subtype 2. None of the non-tumoral nasopharyngeal tissue expressed somatostatin receptors (4).
The first reported imaging only happened 6 years later in 2008 by Bennink et al. It occurred rather serendipitously when a 60-year-old North African lady presented with headaches and diplopia. Initial computed tomography (CT) and magnetic resonance imaging (MRI) scans that were performed suggested differentials of a meningioma or a chordoma hence an Indium-111 octreotide scan was performed to help differentiate the two as meningiomas are known to express somatostatin receptors. Octreoscan showed uptake in the primary base of skull lesion and an avid cervical lymph node. Biopsies of the primary tumour and the lymph node revealed an unsuspected diagnosis of nasopharyngeal carcinoma with a cervical lymph node metastasis (5).
Schartinger et al. subsequently reported in 2015, five patients with NPC that had undergone Gallium-68-DOTA-Octreotide positron emission tomography (PET) scan. They had found in an earlier study of head and neck squamous cancers that NPC showed much higher somatostatin receptor avidity compared to the other head and neck squamous cancers. Four out of five of their NPC patients showed high tracer uptake (SUVmax: 9.2–17.1) with one showing low uptake (SUVmax: 3.6) (6).
Khor et al. reported a comparative study between fluorodeoxyglucose (FDG)-PET and DOTA-NOC [DOTA0-(1NAI3) Octreotide] PET in four NPC patients. There was generally good concordance between the two tracers. There was however discordance in the case of recurrent NPC with the recurrent tumor showing greater FDG uptake but low DOTA-NOC uptake. The authors also noted that DOTA-NOC PET/CT may be useful for assessing intracranial involvement as there is less background physiological brain parenchymal uptake. It may also be better at differentiating reactive lymph nodes (7).
The presence of somatostatin receptors in NPC opens up exciting opportunities for the use of PRRT. These patients have few treatment options available and peptide receptor radiotherapy is an attractive option being able to selectively target tissues overexpressing the somatostatin receptor. Further clinical trials are needed to evaluate the effectiveness of this novel treatment which has become standard in gastroenteropancreatic neuroendocrine tumors.
Activation of SSTR activates several downstream pathways
Somatostatin receptors are G-protein coupled receptors. Signaling through somatostatin receptors involves binding of somatostatin or cortistatin to its various receptor subtypes (sstr1–5) in the context of auto-, para- or endocrine stimulation. Binding of these ligands to somatostatin receptors induces G-protein activation and downstream activation of various pathways. The activities of several key enzymes, including adenylyl cyclase, phosphotyrosine phosphatases (PTPases) and mitogen activated protein kinase (MAPK) are modulated by somatostatin receptor G-protein activation. In addition, calcium and potassium channels and the sodium-proton antiporter respond to somatostatin receptor activation and cause changes in the intracellular levels of calcium and potassium ions. In most cells, Ca2+ signaling is downregulated by somatostatin receptor activation owing to the inhibition of calcium channels and intracellular Ca2+ release or the activation of K+ channels, which results in membrane hyperpolarization. The resultant effect of these is a decrease in hormone secretion. The downstream activation of PTPases can result in inhibition of cell growth and increase apoptosis.
All known human somatostatin receptors can inhibit adenylyl cyclase and decrease cyclic adenosine monophosphate (cAMP) levels. This affects various downstream elements, in particular protein kinase A. This in turn acts as an activator of cAMP response-element-binding protein. Somatostatin receptor subtypes can be coupled to various phospholipase C (PLC) isoforms. In certain systems, somatostatin receptor activation increases the enzymatic activity of PLCβ2 and PLCβ3, and hence the intracellular levels of inositol trisphosphate (IP3) and Ca2+ (3,8).
From imaging to therapy
The concept of “theranostics” (combination of therapeutics and diagnostics) was coined by the US consultant John Funkhouser, to describe a material that allows the combined diagnosis, treatment and follow up of a disease. This approach allows the selection of a sub-population of patients most likely to benefit from a targeted therapy in accordance with their “molecular profile” at a given time-point, or, conversely, those patients for whom the risk of adverse effects is higher (9). This has been applied successfully for neuroendocrine tumors and has the potential in metastatic NPC, to select patients which may benefit from PRRT (Figure 1).
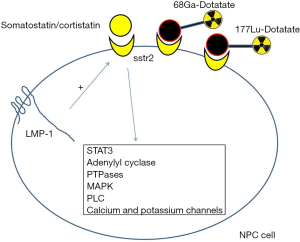
Treatment with radionuclide labeled peptides has shown promising results in neuroendocrine tumors. In the recently reported NETTER-1 trial, it was shown that PRRT with Lu-177 showed significant improvement in progression-free survival (PFS) and overall survival (OS). The estimated rate of PFS at month 20 was 65.2% [95% confidence interval (CI): 50.0–76.8] in the 177Lu-Dotatate group and 10.8% (95% CI: 3.5–23.0) in the control group. The median PFS had not yet been reached in the 177Lu-Dotatate group and was 8.4 months (95% CI: 5.8–9.1) in the control group (hazard ratio for disease progression or death with 177Lu-Dotatate vs. control group, 0.21; 95% CI: 0.13–0.33; P<0.001), which represented a 79% lower risk of disease progression or death in the 177Lu-Dotatate group than in the control group. Consistent treatment benefits associated with 177Lu-Dotatate were observed irrespective of stratification factors and prognostic factors, which included levels of radiotracer uptake on somatostatin receptor scintigraphy, tumor grade, age, sex, and tumor marker levels. In a planned interim analysis of OS, a total of 14 deaths in the 177Lu-Dotatate group and 26 deaths in the control group were observed. This represented an estimated risk of death that was 60% lower in the 177Lu-Dotatate group than in the control group (hazard ratio for death with 177Lu-Dotatate group vs. control group, 0.40; P=0.004) (10). The possibility of PRRT in NPC is promising and requires further investigation.
In localized and recurrent NPC, somatostatin receptor imaging may assist in radiotherapy target delineation and planning. In particular, in regions around the brain where there is high physiological FDG uptake, somatostatin receptor imaging may help improve the delineation of at risk areas and also allow for a higher radiation boost to be given to these areas.
Conclusions
Somatostatin receptor imaging sheds new insight into the biology of NPC and opens up exciting and novel diagnostic and therapeutic opportunities in the management of NPC.
Acknowledgments
Funding: This work is supported by the National Cancer Center Research Fund (NCCRF-YR2015-JAN-PG1).
Footnote
Provenance and Peer Review: This article was commissioned by the Editor-in-Chief (Melvin Lee Kiang Chua) for the series “Inaugural Issue” published in Annals of Nasopharynx Cancer. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/anpc.2018.01.01). The series “Inaugural Issue” was commissioned by the editorial office without any funding or sponsorship. KS serves as an unpaid editorial board member of Annals of Nasopharynx Cancer. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Taniyama Y, Suzuki T, Mikami Y, et al. Systemic distribution of somatostatin receptor subtypes in human: an immunohistochemical study. Endocr J 2005;52:605-11. [Crossref] [PubMed]
- Keskin O, Yalcin S. A review of the use of somatostatin analogs in oncology. Onco Targets Ther 2013;6:471-83. [PubMed]
- Weckbecker G, Lewis I, Albert R, et al. Opportunities in somatostatin research: biological, chemical and therapeutic aspects. Nat Rev Drug Discov 2003;2:999-1017. [Crossref] [PubMed]
- Loh KS, Waser B, Tan LK, et al. Somatostatin receptors in nasopharyngeal carcinoma. Virchows Arch 2002;441:444-8. [Crossref] [PubMed]
- Bennink RJ, van der Meulen FW, Freling NJ, et al. Somatostatin receptor scintigraphy in nasopharyngeal carcinoma. Clin Nucl Med 2008;33:558-61. [Crossref] [PubMed]
- Schartinger VH, Dudás J, Url C, et al. (68)Ga-DOTA (0)-Tyr (3)-octreotide positron emission tomography in nasopharyngeal carcinoma. Eur J Nucl Med Mol Imaging 2015;42:20-4. [Crossref] [PubMed]
- Khor LK, Loi HY, Sinha AK, et al. (68)Ga-DOTA-peptide: A novel molecular biomarker for nasopharyngeal carcinoma. Head Neck 2016;38:E76-80. [Crossref] [PubMed]
- Patel YC. Somatostatin and its receptor family. Front Neuroendocrinol 1999;20:157-98. [Crossref] [PubMed]
- Idée JM, Louguet S, Ballet S, et al. Theranostics and contrast-agents for medical imaging: a pharmaceutical company viewpoint. Quant Imaging Med Surg 2013;3:292-7. [PubMed]
- Strosberg J, El-Haddad G, Wolin E, et al. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N Engl J Med 2017;376:125-35. [Crossref] [PubMed]
Cite this article as: Nei WL, Jain A, Wang FQ, Khor LK, Yeong JPS, Han ST, Sommat K, Ang MK, Soong YL, Loke KSH. Somatostatin receptor imaging in nasopharyngeal cancer. Ann Nasopharynx Cancer 2018;2:2.


