
Nasopharyngeal carcinoma contouring guidelines—the first step in a thousand mile journey
“The journey of a thousand miles begins with one step.”—Lao Tzu (NOT Confucius).
Contouring consensus guidelines for head and neck carcinomas have been available as a useful tool for the radiation oncologist from various known experts and cooperative groups around the globe (1). However, nasopharyngeal carcinoma (NPC) is a disease unique in its epidemiology and behavior, necessitating separate guidelines for this distinctive head and neck malignancy.
Lee and her co-authors made a valiant first attempt in reaching and publishing a consensus amongst regional and global experts in radiation oncology regarding the most appropriate volumes and dose levels for the disease (2). Notable in the publication is substantial disagreement amongst the experts on various aspects of volume and dose prescription. The disagreement amongst the expert panel is not unexpected as the contouring and dose prescription protocols in their respective centres have shown impressive numbers in terms of survival, control and risks for severe toxicity.
At our institute, like in most other Asian centers, we include the entire nasopharynx in the high-risk CTV (GR-CTV, or CTVp1). The consensus defines the nasopharynx as just the mucosa and not including the pre-vertebral muscles, unless involved. Inclusion of this entire structure should not add toxicity and should be more in-keeping with the definition of HR-CTV. Even in the absence of evidence of abnormalities on MRI, the entire nasopharynx is in theory at high-risk of occult disease (field cancerization) as is the case for cervical cancer, for which the entire cervix is considered part of the HR-CTV. Furthermore, similar to the junction line that exists in the cervix, there exists an intermediate pseudostratified cuboidal type that is a transition between the pseudostratified columnar epithelium and the squamous epithelium. This intermediate epithelium is most susceptible to carcinogenesis, thus the propensity of NPC to grow in the lateral walls, posterior wall and anterior walls, in that order, corresponding to the predominance of this intermediate epithelium (3). The caudal border should theoretically cover this transition zone, which may vary among individuals. The C1 or level of the soft palate seems reasonable and coincidentally, this is included within the usual prescription isodose volume in intracavitary brachytherapy (ICBT) (4) which is given as a boost in some Asian centers. Less margin is thus necessary anteriorly and should enable us to spare the soft palate.
The 5+5 mm expansion as proposed by the DAHANCA guidelines and also basically agreed upon by other trial groups (5,6) may not be unreasonable for the intermediate-risk CTV (IR-CTV, or CTVp2), but we have to agree that NPC is biologically different from other HNSCC and may need some refinements in redefining the extent which requires a higher radiation dose. In this era of personalisation of treatment and high tech “dose-painting” and adaptive radiotherapy (RT), more accurate identification and delineation of areas at high risk of local failure is brought more to the forefront. Also, the predisposition for NPC for perineural spread as well as the exquisite radiosensitivity of the tumor makes it necessary for radiation oncologists to think outside the “5+5” paradigm when treating NPC or to find suitable adaptations for the disease.
Local failure is either marginal or within the high-dose region. The objective of standardisation of target delineation is to decrease the incidence of marginal and geographic misses and hopefully, the evolution of these guidelines will allow for a more thorough understanding of the patterns of spread of NPC. However, a good number of failures still occur within the high-dose region. It is important to likewise look at certain prognostic indices and biomarkers to define a population of NPC patients who may benefit from trials of dose escalation. A paper published at the JCO may provide the initial clues to radioresistance of some patients with NPC (7). In a similar light, it is imperative to dichotomize in future studies the “true failures” which herald truly resistant disease and differentiate them from the marginal failures, which may be a result of inadequate tumor coverage in either the CTVp1 or the CTVp2. The former should occur in the high dose region and should have received as little treatment breaks as possible and with an acceptable cumulative cisplatinum dose. The crux of the issue is how little a treatment break can be labeled “reasonable” and what is the “acceptable” cumulative level of cisplatinum. Our centre implements keeping overall treatment time for radiotherapy at a maximum of 7.5 (1 week maximum cumulative treatment break) and attempts to achieve 200 mg/m2 of concurrent cisplatin in all patients. Induction chemotherapy is only given upon consensus by the NPC tumor board that the risk of distant metastasis warrants the delivery of ICT.
There also appears to be no recommendations for low-risk CTV (LR-CTV) such as coverage above the skull base as the most superior extent of the recommendations mention up to the foramina ovale, rotundum, lacerum and petrous tip. Too stringent coverage criteria may present a risk for intracranial failure via the perineural route. Perhaps the traditional volumes covered in the seminal work by Ho (8) wherein the fields covered potential intracranial extension by bringing the parallel opposed fields 10–15 mm above the sella turcica and coning down after 54–60 Gy. Indeed, our series show penumbral failures at or above the skull base, indicating the presence of subclinical perineural spread despite the absence of intracranial spread in pre-RT evaluations (Figure 1). The patients with the latter failures received their definitive treatment in low-volume, community centers, albeit following standard treatment guidelines, commonly the National Comprehensive Cancer Network guidelines.
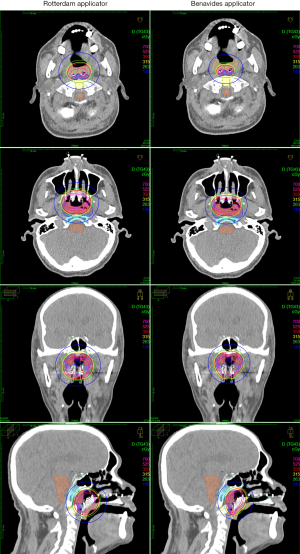
Indeed, even in the era of intensity-modulated radiotherapy and chemoradiation (CRT) with excellent local control rates (up to 85.8% overall 8-year local failure-free survival; T1: 91.7%, T2: 88.2%, T3: 87.2%; T4, 71.6%) have been achieved (9). NPC recurrences within high-dose regions occur, indicating radioresistance. The benefit of dose escalation has been demonstrated even in the era of CRT (10) and some centers in Asia continue to give ICBT boost in order to dose-escalate in AJCC 7 T1–T2 disease (11,12). The guideline is reassuring for centers that do ICBT boost, in that the CTVp1 and CTVp2 should be well-covered by the 350 and 140 cGy isodose volumes if ICBT is given in 350 cGy fractions twice-daily (see photo) (4).
Finally, there have been publications showing that outcomes in head and neck cancers appear to be better when these patients are treated in higher volume academic centers by high volume, specialists (13,14). It appears that while the expert panels and the institutions they treat in meet the criteria separately mentioned by Corry and David, the outcomes would intuitively be different amongst different institutions with different levels of subspecialist expertise, even if the contouring and prescription guidelines were followed to the letter where patients treated in high volume centers by high volume specialists may have better outcomes than those treated in lower volume centers by general radiation oncologists. These findings remind us that while these guidelines serve to standardize target delineation and dose prescription, good outcomes result from sound clinical judgment, good and collaborative clinical practice. Radiation oncologists will have to work collaboratively with radiologists for accurate GTV delineation, with medical physicists and radiation technologists towards accurate treatment delivery, and with the medical oncologist and the patient himself, for proper management of treatment toxicity and thus compliance.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Nasopharynx Cancer. The article did not undergo external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/anpc.2018.04.05). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Grégoire V, Evans M, Le QT, et al. Delineation of the primary tumour Clinical Target Volumes (CTV-P) in laryngeal, hypopharyngeal, oropharyngeal and oral cavity squamous cell carcinoma: AIRO, CACA, DAHANCA, EORTC, GEORCC, GORTEC, HKNPCSG, HNCIG, IAG-KHT, LPRHHT, NCIC CTG, NCRI, NRG Oncology, PHNS, SBRT, SOMERA, SRO, SSHNO, TROG consensus guidelines. Radiother Oncol 2018;126:3-24. [Crossref] [PubMed]
- Lee AW, Ng WT, Pan JJ, et al. International guideline for the delineation of the clinical target volumes (CTV) for nasopharyngeal carcinoma. Radiother Oncol 2018;126:25-36. [Crossref] [PubMed]
- Batsakis JG. The pathology of head and neck tumors: nasal cavity and paranasal sinuses, part 5. Head Neck Surg 1980;2:410-9. [Crossref] [PubMed]
- Bacorro WR, Agas RF, Cabrera SR, et al. A novel applicator design for intracavitary brachytherapy of the nasopharynx: simulated reconstruction, image-guided adaptive brachytherapy planning and dosimetry. Brachytherapy 2018. Available online: https://doi.org/
10.1016/j.brachy.2018.03.004 - NRG-HN001. Randomized phase II and phase III studies of individualized treatment for nasopharyngeal carcinoma based on biomarker Epstein Barr Virus (EBV) Deoxyribonucleic Acid (DNA). Available online: https://www.nrgoncology.org/Clinical-Trials/Protocol-Table
- Ng WT, Lee MC, Hung WM, et al. Clinical outcomes and patterns of failure after intensity-modulated radiotherapy for nasopharyngeal carcinoma. Int.J.Radiat Oncol Biol Phys 2011;79:420-8. [Crossref] [PubMed]
- Li YQ, Tian YM, Tan SH, et al. Prognostic Model for Stratification of Radioresistant Nasopharynx Carcinoma to Curative Salvage Radiotherapy. J Clin Oncol 2018;36:891-9. [Crossref] [PubMed]
- Ho J. Nasopharynx. In: Halnan K. editor. Treatment of Cancer. New York: Igaku-Shoin: Chapman and Hall, 1998;928.
- Au KH, Ngan RK, Ng AW, et al. Treatment outcomes of nasopharyngeal carcinoma in modern era after intensity modulated radiotherapy (IMRT) in Hong Kong: A report of 3328 patients (HKNPCSG 1301 study). Oral Oncol 2018;77:16-21. [Crossref] [PubMed]
- Levendag PC, Lagerwaard FJ, Noever I, et al. Role of endocavitary brachytherapy with or without chemotherapy in cancer of the nasopharynx. Int J Radiat Oncol Biol Phys 2002;52:755-68. [Crossref] [PubMed]
- Wu J, Guo Q, Lu JJ, et al. Addition of intracavitary brachytherapy to external beam radiation therapy for T1-T2 nasopharyngeal carcinoma. Brachytherapy 2013;12:479-86. [Crossref] [PubMed]
- Chao HL, Liu SC, Tsao CC, et al. Dose escalation via brachytherapy boost for nasopharyngeal carcinoma in the era of intensity-modulated radiation therapy and combined chemotherapy. J Radiat Res 2017;58:654-60. [Crossref] [PubMed]
- Corry J, Peters LJ, Rischin D. Impact of center size and experience on outcomes in head and neck cancer. J Clin Oncol 2015;33:138-40. [Crossref] [PubMed]
- David JM, Ho AS, Luu M, et al. Treatment at high-volume facilities and academic centers is independently associated with improved survival in patients with locally advanced head and neck cancer. Cancer 2017;123:3933-42. [Crossref] [PubMed]
Cite this article as: Mejia MBA, Bacorro WR, Sogono PG. Nasopharyngeal carcinoma contouring guidelines—the first step in a thousand mile journey. Ann Nasopharynx Cancer 2018;2:8.


