Hypothyroidism after radiotherapy for nasopharyngeal carcinoma
Introduction
Nasopharyngeal carcinoma (NPC) is a unique type of head-and-neck cancers (HNC). Differences from other cancers in head and neck region include (I) definite radiotherapy (RT) and/or chemotherapy is the primary treatment, (II) intensity-modulated radiotherapy (IMRT) is considered standard treatment technique, (III) pituitary gland is partially included in the radiation field, (IV) surgical neck dissection involving thyroid gland is not performed, and (V) long survival outcome is expected. Despite advance RT techniques, treatment causes unavoidable irradiation to normal organs (e.g., thyroid gland, salivary glands, mandible, spinal cord) which leads to radiation-induced toxicities and affects long-term quality of life (QoL) in NPC survivors.
Radiation-induced hypothyroidism (RIHT) is the most common late effect of thyroid gland after RT to the neck. Incidence of RIHT ranged from 23–53% in HNC (1-4), and 14–54% in NPC (5-15). RIHT is observed as a late adverse event and usually occurs within 2 years after treatment. Without comprehensive follow-up, delayed diagnosis of RIHT results in diminished QoL and increases risk of life-threatening conditions like ischemic heart disease, myxedema, and birth defect (16-18).
Although evidences suggested a dose-response relationship between thyroid dosimetry and risk of RIHT (6,8,12,13), currently, there has been no consensus for dose constraint of thyroid gland, even in the latest Quantitative Analysis of Normal Tissue Effects in the Clinic (QUANTEC) guideline (19). This review summarizes the pathophysiologic changes of thyroid gland after radiation and recommendations of thyroid constraint from literatures as well as predictive tools for predicting RIHT in NPC.
Pathophysiology of thyroid gland
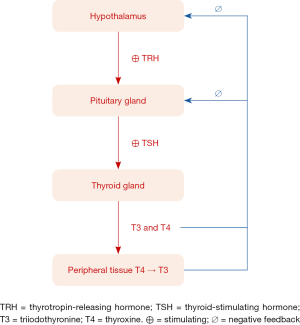
Thyroid gland is a vital endocrine organ that produces and releases thyroid hormones [triiodothyronine (T3) and thyroxine (T4)] which help regulate metabolism, growth and development of human body. Other important organs that maintain thyroid homeostasis are hypothalamus and pituitary gland, known as hypothalamic–pituitary–thyroid (HPT) axis. Hypothalamus secretes thyrotropin-releasing hormone (TRH) which stimulates pituitary gland to produce thyrotropin or thyroid-stimulating hormone (TSH). The TSH stimulates thyroid gland to produce and release thyroid hormones to reach normal level in blood circulation. In normal situation, thyroid gland releases small amounts of T3, an active form of thyroid hormone, and large amount of T4, prohormone, which is converted to T3 in peripheral tissues. Then T3 and T4, in turn, causes negative feedback control to hypothalamus and pituitary gland. Consequently, this loop-feedback mechanism maintains equilibrium concentrations for TRH, TSH and thyroid hormones (Figure 1).
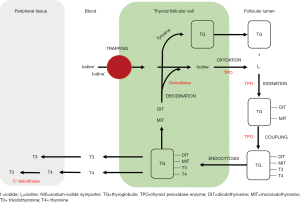
Thyroid gland is sensitive to RT. Many radiation-induced thyroid disorders have been reported including hypothyroidisms and hyperthyroidisms, Hashimoto thyroiditis, Graves’ disease, and thyroid tumors (2,20). Primary hypothyroidism is the most common thyroid disorder after head-and-neck RT, but actual mechanism is still clearly unknown. Radiation causes damages to follicular epithelial cells, parenchymal cells and small blood vessels of thyroid gland, leading to reduction of thyroid mass (6,13). Other causations include capsular fibrosis and iodine insufficiency due to malnutrition. Also, it is speculated that immune-mediated reaction is one of the common processes (2,8). In NPC patients treated with RT, there was abnormal rise of thyroid antibodies, thyroperoxidase antibody (anti-TPO) and thyroglobulin antibody (anti-TG), which were indicators of thyroiditis from autoimmune response (8). Under normal circumstances, TPO and TG are enzyme and glycoprotein that involve in the synthesis of thyroid hormones (Figure S1).
Diagnosis of hypothyroidism
Diagnosis of hypothyroidism requires laboratory tests, i.e., TSH, (free) T3 and (free) T4, with or without clinical signs/symptoms. Clinical or overt hypothyroidism is generally defined as high serum TSH level with low T4 and presence of clinical presentations of hypothyroidism including generalized symptoms (fatigue, weakness, cold intolerance, weight gain, dry skin, edema, constipation, menorrhagia), neuromuscular symptoms (myalgia, paresthesia, arthralgia, cognitive dysfunction, impaired consciousness) and psychiatric symptoms (depression). Subclinical or biochemical hypothyroidism is usually defined as increased serum TSH level and low/normal T4 without symptoms. In normal population, clinical hypothyroidism is found about 1–2% and more common in older women (21). The prevalence of subclinical hypothyroidism is about 6% and associated with an increased risk of coronary heart disease and mortality in patients with high serum TSH concentration (≥10 mIU/L) (17,18). Therefore, early thyroid hormone replacement therapy, e.g., levothyroxine, is recommended in those individuals with subclinical hypothyroidism and serum TSH concentration ≥10 mIU/L. After radiation treatment, approximately 10% and 30% of patients developed clinical and subclinical hypothyroidism, respectively, and about one-fourth in the latter group developed clinical symptoms later with a median time of 11 months (9). Noting that, given radiation dose to pituitary gland in NPC patients with T3 (base of skull involvement) and T4 (intracranial involvement), central hypothyroidism can also occur and is characterized as low TSH and T4 (7,14).
Radiation-induced hypothyroidism
Changes of thyroid glands after radiation
In NPC, cervical lymph nodes are usually involved, therefore, it is recommended to regularly include bilateral neck lymphatics in the clinical target volumes (22). As thyroid gland is a normal structure located in lower neck region, neck irradiation causes inevitable dose to the thyroid gland.
Several studies reported dose-response relationship between mean thyroid dose and glandular volume reduction (6,13) as well as hormonal changes (6,8,12,13). Longitudinal studies on the pattern of thyroid gland changes after RT in NPC patients found that most changes occurred in the first 2 years. With the mean pre-treatment thyroid volume of approximately 18 cm3 in Lin studies, there was thyroid volume loss about 20%, 30% and 40% at 6, 12 and 30 months, respectively (6,13). There was a decrease of free T4 level starting at 6 months after RT, obvious drop at 12-month interval, reaching 20% reduction at 30-month interval, and became steady after 36 months. Similarly, serum free T3 level showed slightly decreased at 6 months but relatively remained steady throughout the study period. In contrast, the TSH level showed a rising trend gradually after completion of RT and the greatest decrease at 6–12 months up to 24 months, followed by a decreasing trend (6,8,13). The possible explanation for the phenomenon was the shrinkage of thyroid gland, caused by radiation damage and autoimmune reaction, occurred in the first 6 months and led to reduced production of thyroid hormones. Based on feedback mechanism, the reduction of circulating free T4 triggered pituitary gland to secrete more TSH to stimulate thyroid gland to produce more thyroid hormones until reaching normal level in the blood circulation. Evidences also showed that the thyroid gland and function were partially recovered after 2–3 years but long follow-up study was still needed on complete recovery ability of thyroid gland (13).
Factors associated with RIHT
In pre-IMRT era. Emami found that clinical hypothyroidism was associated with damage to large fraction of the whole thyroid gland (2/3 or more). Irradiation to the whole gland volume at 45 Gy resulted in the estimated probability of 8% clinical hypothyroidism at 5 years (tolerance dose (TD) 8/5). The estimated TD 13/5 and 35/5 were 60 and 70 Gy, respectively (23). But no TD for subclinical hypothyroidism was reported.
Currently, IMRT, an inverse treatment planning algorithm that maximizes dose conformity to tumor while minimizing unnecessary dose to surrounding critical structures, is considered as a standard RT technique for NPC in view of both dosimetric and clinical benefit (24-27). However, the benefit of IMRT comes with a potential drawback. With no specific dose constraint during plan optimization, IMRT contributed to a higher dose and irradiated volume of thyroid gland which might result in higher incidence and shorter latency of RIHT, compared with three-dimensional conformal radiotherapy (3D-CRT) in which midline shield in the anterior lower neck field usually applied (3).
With attempts to define threshold dose to the thyroid gland, a systematic review of 5 dosimetric studies based on RIHT was performed. They analyzed the relationship between the occurrence of RIHT and thyroid gland volume absorbing 10–70 Gy (V10–V70), mean dose (Dmean), minimal dose (Dmin), maximum dose (Dmax) to the thyroid gland. However, the results failed to define a clear threshold dose and dose-volume effect between thyroid dose distribution and the incidence of RIHT due to heterogeneity among studies including study design and parameters (28).
Apart from dosimetric factors, another systematic review and meta-analysis from 37 articles showed that significant clinical risk factors were female gender [Odds ratio (OR) =1.57; 95% confidence interval (CI), 1.30–1.88], partial or hemithyroidectomy (OR =8.28; 95% CI, 5.71–12.02), and Caucasian vs. African American descent (OR =4.84; 95% CI, 2.76–8.49). By fitting dose-response functions using logistic model from data of 4 dosimetric studies, the author showed a dose-response effect with a 50% risk of RIHT at mean thyroid dose of 45 Gy (95% CI, 28–62 Gy) (TD50 =45 Gy) (29). According to IMRT studies in NPC, mean thyroid dose was ranging from 43-54 Gy resulting in high incidence of RIHT up to 54% (12,13,30,31). Thus far, no consensus for IMRT dose constraint of thyroid gland has been established.
However, it is worth noting that there was no NPC study in the first dosimetric systematic review and only 2 NPC studies included in the second systematic review. In this review, Table 1 summarized clinical and dosimetric predictors for RIHT in NPC and recommendations for dose constraint of thyroid gland from the reported literatures. And, other than using only one or two dosimetric constraints, multiple points of dose volume constraint should be concerned during the process of IMRT plan optimization. Figure 2 demonstrated the recommended cut points in treatment planning software. The biological optimization such as equivalent uniform dose (EUD) is beyond the scope of this review.
Table 1
| Study | N | Median follow-up (year) | Treatment | RIHT definition | Incidence of RIHT | Significant predictor (s)* | Suggestions by authors |
|---|---|---|---|---|---|---|---|
| Ulger et al., 2007 (5) | 85 | 2.8 | Conventional RT | ↑TSH, ↔ or ↓FT4 | 14% | Age | – |
| Lee et al., 2016 (9) | 149 | 3.1 | IMRT | ↑TSH or ↓FT4 | 30.9%, 1Y =5.3%, 2Y =29.6%, 3Y =36.2% |
thyroid volume*, thyroid Dmin, Dmean, D5, V30, V35, V40, V45, VS30, VS40, VS45*, VS60* | Thyroid VS45 ≥5 cm3; Thyroid VS60 ≥10 cm3 |
| Luo et al., 2017 (30) | 164 | 2 | 3D-CRT + IMRT 80.5% | ↑TSH | 23.2%, 1Y =14.3%, 2Y =24.6% |
gender*, chemotherapy, thyroid Dmin, Dmean, V25, V30, V35, V40, V45, V50*, V55, V60 | Nomogram (gender, chemotherapy, V50) |
| Sommat et al., 2017 (10) | 102 | 4.1 | IMRT | ↑TSH, ↔ or ↓FT4 | 43.1%, 1Y =33%, 2Y =44.5% |
younger age*, early T stage*, thyroid volume, thyroid Dmin, V40*, V45, V50, Pituitary Dmax | Thyroid V40 ≤85% |
| Zhai et al., 2017 (11) | 135 | 2.8 | IMRT | ↑TSH, ↔ or ↓FT4 | 28.9%, 1Y =13.2%, 2Y =39.9%, 3Y =53.5% |
female, younger* age, thyroid volume, thyroid Dmin, Dmean*, V35, V40, V45*, V50*, thyrotoxicosis | Thyroid V45 ≤50%; Thyroid V50 ≤35% |
| Lin et al., 2018 (13) | 56 | 2 | IMRT | ↑TSH, ↔ or ↓FT4 | 28.6% | Dmean*, D50 | Thyroid Dmean <43 Gy |
| Lertbutsayanukul et al., 2018 (12) | 178 | 3.5 | IMRT | ↑TSH, ↔ or ↓FT4 | 53.9% | female, thyroid volume, pretreatment TSH*, VS60*, | Thyroid VS60 ≥10 cm3 |
| Xu et al., 2018 (14) | 52 | 1.4 | IMRT | ↑TSH, ↔ or ↓FT4; ↓TSH, ↓FT4 | 53.8%, 1Y =33%, 2Y =51%, 3Y =71% |
female, Dmean, V50, thyroid volume | Thyroid Dmean <51.6 Gy; Thyroid V50 <54.5% |
| Luo et al., 2018 (31) | 174 | 2 | 3D-CRT + IMRT 81.6% | ↑TSH, ↔ or ↓FT4 | gender*, Dmin, Dmean, V30, V35, V40, V45, V50*, V55, V60, chemo*, Pituitary Dmax* | Logistic regression model (Table 2) | |
| Huang et al., 2019 (15) | 345 | 3.8 | IMRT | ↑TSH, ↔ or ↓FT4 | 44.1%, 1Y =10.7%, 2Y =25.8%, 3Y =36.3% |
age, gender, nodal status, thyroid volume*, Dmean, Dmin, V25, V35, V45 | Thyroid V25 ≤60%; Thyroid V35 ≤55%; Thyroid V45 ≤45% |
*, significant variables in multivariate analysis. RIHT, radiation-induced hypothyroidism; RT, radiotherapy; 3D-CRT, three-dimensional conformal radiotherapy; IMRT, intensity-modulated radiotherapy; TSH, thyroid-stimulating hormone; FT4, free thyroxine; Dmin, minimal dose; Dmean, mean dose; Dmax, maximal dose; Dx, dose that volume x% of thyroid gland received; Vx, volume receiving dose x Gy; VSx, volume-sparing dose x Gy.
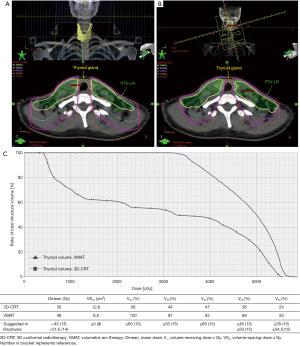
Considering there are many unmodifiable factors, e.g., gender, age, thyroid volume, and pretreatment TSH levels, minimizing unnecessary radiation dose to the thyroid gland is of paramount. This could be accomplished by many approaches, for example (I) matching the IMRT plan to a conventional anterior low-neck field with midline shield, so-called split-filed IMRT (36), (II) omitting treatment of lymph node level IV in T1 disease (37,38), and (III) diminishing overlapping between low-risk planning target volume (PTV-LR) and thyroid gland. Figure 3 demonstrates dose distribution and contour of thyroid gland and PTV-LR in stage T2N2M0 NPC patient. Of note, with minimal overlapping of thyroid and PTV-LR, 40 Gy of isodose line encompassed nearly half of thyroid gland.
Predictive models for RIHT
Although there is no QUANTEC report that covers RIHT, there has recently been proposing the use of normal tissue complication probability (NTCP) model to estimate risk of complication. Furthermore, it has been an increasing interest to evaluate and compare treatment modalities, such as x-ray vs. proton therapy, using NTCP model-based approach (39,40).
Purpose of NTCP model is translating dosimetric input into a clinically relevant outcome. In other words, “irradiated dose to organ” is depicted as “a probability or risk of developing toxicity of that organ”. Various mathematical models have been proposed to estimate the risk of complication as a function of assorted parameters of the treatment plan, tissue architecture and radiosensitivity (33). The most common types of NTCP models are Lyman model, logistic model, and logistic regression model.
Although there were many studies on dose-response model for predicting RIHT in HNC (19,29,32,34,35,41) but they excluded NPC patients or those whose pituitary gland dose >40 Gy due to possible damage to the HPT axis. To dates, there have been only two studies focusing on development of predictive tools for RIHT in NPC patients (30,31). Table 2 summarizes NTCP models for RIHT endpoint in overall HNC.
Table 2
| Study | N | RIHT definition | Time point | Significant non-dosimetric factors | Model and parameters (95% CI) |
|---|---|---|---|---|---|
| Vogelius et al., 2011 (29) | Meta-analysis of 4 studies | ↑TSH | between 1–11 y | Logistic model | |
| D50, thyroid mean dose =45 [28,62] | |||||
| γ 50, thyroid mean dose =1.40 [0.50,2.20] | |||||
| AUC =N/A | |||||
| Bakhshandeh et al., 2013 (32) | 65 HNC (NPC=11) | CTCAE version 4.02 | within 1 y after RT | Lyman EUD mean dose model | |
| Grade 1 (↑TSH, ↔ FT4) or higher | D50, thyroid mean dose =60 [56,73] | ||||
| m thyroid mean dose =0.27 [0.20,0.39] | |||||
| AUC=N/A | |||||
| Boomsma et al., 2012 (33) | 105 HNC | ↑TSH, ↔ or ↓FT4 | within 2 y after RT | Thyroid volume | Logistic regression model |
| β thyroid mean dose =0.062 [0.029,0.096] | |||||
| β thyroid volume =−0.19 [−0.30, −0.08] | |||||
| Constant [β0] =0.011 | |||||
| AUC =0.85 [0.78, 0.92] | |||||
| Cella et al., 2012 (34) | 53 HL | ↑TSH, ↓FT3 or ↓FT4 | median follow-up 2.7 y | Thyroid volume, gender | Logistic regression model |
| β thyroid V30 [cc] =0.26 [0.08,0.44] | |||||
| β thyroid volume =−0.27 [−0.49, −0.05] | |||||
| β gender =−2.21 [−3.88,−0.54] | |||||
| Constant [β0] =1.94 | |||||
| AUC =0.87 [0.75, 0.95] | |||||
| Rønjom et al., 2015 (35) | 198 HNC | ↑TSH, ↔ or ↓FT4 | within 2.1 y after RT | Thyroid volume | Logistic regression model |
| β thyroid mean dose =0.18 [0.10,0.37] | |||||
| β thyroid volume =−0.30 [−0.62,−0.15] | |||||
| Constant [β0] =−4.92 | |||||
| AUC =N/A | |||||
| Luo et al., 2017 (30) | 164 NPC | ↑TSH | Median, follow-up 2 y | Gender, chemotherapy | Nomogram |
| Luo et al., 2018 (31) | 174 NPC | ↑TSH, ↔ or ↓FT4 | Median, follow-up 2 y | Gender, chemotherapy | β thyroid V50 =0.050 |
| β pituitary Dmax =−0.026 | |||||
| β gender =1.280 | |||||
| β chemotherapy =2.902 | |||||
| Constant [β0] =−2.695 | |||||
| AUC =0.79 [0.73, 0.85] | |||||
| β thyroid V50 =0.050 |
RIHT, radiation-induced hypothyroidism; HNC, head-and-neck cancer; HL, Hodgkin’s lymphoma; NPC, nasopharyngeal carcinoma; RT, radiotherapy; TSH, thyroid-stimulating hormone; FT3, free triiodothyronine; FT4, free thyroxine; EUD, equivalent uniform dose; CI, confidence interval; AUC, area under the receiver operating curve; N/A, not applicable.
Most studies were retrospective and confounded with heterogeneity of disease and patient characteristics, variety of treatment techniques/regimens, and different follow-up protocol among studies. These limitations inevitably affected the predictive power of model (42). Therefore, prior to clinical application, it is necessary to perform external validation, especially in different clinical entity. More importantly, a well-designed prospective study with long-term follow-up period would accomplish precise and accurate information. Baseline thyroid function test is required to exclude preexisting thyroid dysfunction and not to overestimate the incidence of RIHT after radiation. Due to different fractionation regimen, DVH data of the thyroid gland should be converted to an equivalent dose in 2 Gy/fraction because different parts of the thyroid gland received heterogeneous fractionation dose. Besides, the novel predictive markers including circulating thyroid immune-mediated factors and radiogenomic data have potential to be integrated into the NTCP model for improving prediction ability and further implementing in treatment plan optimization.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/anpc.2020.03.03). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Norris AA, Amdur RJ, Morris CG, et al. Hypothyroidism when the thyroid is included only in the low neck field during head and neck radiotherapy. Am J Clin Oncol 2006;29:442-5. [Crossref] [PubMed]
- Alterio D, Jereczek-Fossa BA, Franchi B, et al. Thyroid disorders in patients treated with radiotherapy for head-and-neck cancer: a retrospective analysis of seventy-three patients. Int J Radiat Oncol Biol Phys 2007;67:144-50. [Crossref] [PubMed]
- Diaz R, Jaboin JJ, Morales-Paliza M, et al. Hypothyroidism as a consequence of intensity-modulated radiotherapy with concurrent taxane-based chemotherapy for locally advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys 2010;77:468-76. [Crossref] [PubMed]
- Sachdev S, Refaat T, Bacchus ID, et al. Thyroid V50 Highly Predictive of Hypothyroidism in Head-and-Neck Cancer Patients Treated With Intensity-modulated Radiotherapy (IMRT) Am J Clin Oncol 2017;40:413-7. [Crossref] [PubMed]
- Ulger S, Ulger Z, Yildiz F, et al. Incidence of hypothyroidism after radiotherapy for nasopharyngeal carcinoma. Med Oncol 2007;24:91-4. [Crossref] [PubMed]
- Lin Z, Wu VW, Lin J, et al. A longitudinal study on the radiation-induced thyroid gland changes after external beam radiotherapy of nasopharyngeal carcinoma. Thyroid 2011;21:19-23. [Crossref] [PubMed]
- Huang S, Wang X, Hu C, et al. Hypothalamic-pituitary-thyroid dysfunction induced by intensity-modulated radiotherapy (IMRT) for adult patients with nasopharyngeal carcinoma. Med Oncol 2013;30:710. [Crossref] [PubMed]
- Lin Z, Chen L, Fang Y, et al. Longitudinal study on the correlations of thyroid antibody and thyroid hormone levels after radiotherapy in patients with nasopharyngeal carcinoma with radiation-induced hypothyroidism. Head Neck 2014;36:171-5. [Crossref] [PubMed]
- Lee V, Chan SY, Choi CW, et al. Dosimetric Predictors of Hypothyroidism After Radical Intensity-modulated Radiation Therapy for Non-metastatic Nasopharyngeal Carcinoma. Clin Oncol (R Coll Radiol) 2016;28:e52-60. [Crossref] [PubMed]
- Sommat K, Ong WS, Hussain A, et al. Thyroid V40 Predicts Primary Hypothyroidism After Intensity Modulated Radiation Therapy for Nasopharyngeal Carcinoma. Int J Radiat Oncol Biol Phys 2017;98:574-80. [Crossref] [PubMed]
- Zhai RP, Kong FF, Du CR, et al. Radiation-induced hypothyroidism after IMRT for nasopharyngeal carcinoma: Clinical and dosimetric predictors in a prospective cohort study. Oral Oncol 2017;68:44-9. [Crossref] [PubMed]
- Lertbutsayanukul C, Kitpanit S, Prayongrat A, et al. Validation of previously reported predictors for radiation-induced hypothyroidism in nasopharyngeal cancer patients treated with intensity-modulated radiation therapy, a post hoc analysis from a Phase III randomized trial. J Radiat Res 2018;59:446-55. [Crossref] [PubMed]
- Lin Z, Yang Z, He B, et al. Pattern of radiation-induced thyroid gland changes in nasopharyngeal carcinoma patients in 48 months after radiotherapy. PloS One 2018;13:e0200310. [Crossref] [PubMed]
- Xu Y, Shao Z, Tang T, et al. A dosimetric study on radiation-induced hypothyroidism following intensity-modulated radiotherapy in patients with nasopharyngeal carcinoma. Oncol Lett 2018;16:6126-32. [PubMed]
- Huang CL, Tan HW, Guo R, et al. Thyroid dose-volume thresholds for the risk of radiation-related hypothyroidism in nasopharyngeal carcinoma treated with intensity-modulated radiotherapy-A single-institution study. Cancer Med 2019;8:6887-93. [Crossref] [PubMed]
- Razvi S, Weaver JU, Vanderpump MP, et al. The incidence of ischemic heart disease and mortality in people with subclinical hypothyroidism: reanalysis of the Whickham Survey cohort. J Clin Endocrinol Metab 2010;95:1734-40. [Crossref] [PubMed]
- Rodondi N, den Elzen WP, Bauer DC, et al. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA 2010;304:1365-74. [Crossref] [PubMed]
- Weiss IA, Bloomgarden N, Frishman WH. Subclinical hypothyroidism and cardiovascular risk: recommendations for treatment. Cardiol Rev 2011;19:291-9. [Crossref] [PubMed]
- Marks LB, Yorke ED, Jackson A, et al. Use of normal tissue complication probability models in the clinic. Int J Radiat Oncol Biol Phys 2010;76:S10-9. [Crossref] [PubMed]
- Hancock SL, McDougall IR, Constine LS. Thyroid abnormalities after therapeutic external radiation. Int J Radiat Oncol Biol Phys 1995;31:1165-70. [Crossref] [PubMed]
- Vanderpump MP, Tunbridge WM. Epidemiology and prevention of clinical and subclinical hypothyroidism. Thyroid 2002;12:839-47. [Crossref] [PubMed]
- Lee AW, Ng WT, Pan JJ, et al. International guideline for the delineation of the clinical target volumes (CTV) for nasopharyngeal carcinoma. Radiother Oncol 2018;126:25-36. [Crossref] [PubMed]
- Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int Int J Radiat Oncol Biol Phys 1991;21:109-22. [Crossref] [PubMed]
- Kam MK, Chau RM, Suen J, et al. Intensity-modulated radiotherapy in nasopharyngeal carcinoma: dosimetric advantage over conventional plans and feasibility of dose escalation. Int J Radiat Oncol Biol Phys 2003;56:145-57. [Crossref] [PubMed]
- Pow EH, Kwong DL, McMillan AS, et al. Xerostomia and quality of life after intensity-modulated radiotherapy vs. conventional radiotherapy for early-stage nasopharyngeal carcinoma: initial report on a randomized controlled clinical trial. Int J Radiat Oncol Biol Phys 2006;66:981-91. [Crossref] [PubMed]
- Kam MK, Leung SF, Zee B, et al. Prospective randomized study of intensity-modulated radiotherapy on salivary gland function in early-stage nasopharyngeal carcinoma patients. J Clin Oncol 2007;25:4873-9. [Crossref] [PubMed]
- Peng G, Wang T, Yang KY, et al. A prospective, randomized study comparing outcomes and toxicities of intensity-modulated radiotherapy vs. conventional two-dimensional radiotherapy for the treatment of nasopharyngeal carcinoma. Radiother Oncol 2012;104:286-93. [Crossref] [PubMed]
- Boomsma MJ, Bijl HP, Langendijk JA. Radiation-induced hypothyroidism in head and neck cancer patients: a systematic review. Radiother Oncol 2011;99:1-5. [Crossref] [PubMed]
- Vogelius IR, Bentzen SM, Maraldo MV, et al. Risk factors for radiation-induced hypothyroidism: a literature-based meta-analysis. Cancer 2011;117:5250-60. [Crossref] [PubMed]
- Luo R, Li M, Yang Z, et al. Nomogram for radiation-induced hypothyroidism prediction in nasopharyngeal carcinoma after treatment. Br J Radiol 2017;90:20160686. [Crossref] [PubMed]
- Luo R, Wu VWC, He B, et al. Development of a normal tissue complication probability (NTCP) model for radiation-induced hypothyroidism in nasopharyngeal carcinoma patients. BMC cancer 2018;18:575. [Crossref] [PubMed]
- Bakhshandeh M, Hashemi B, Mahdavi SR, et al. Normal tissue complication probability modeling of radiation-induced hypothyroidism after head-and-neck radiation therapy. Int J Radiat Oncol Biol Phys 2013;85:514-21. [Crossref] [PubMed]
- Boomsma MJ, Bijl HP, Christianen ME, et al. A prospective cohort study on radiation-induced hypothyroidism: development of an NTCP model. Int J Radiat Oncol Biol Phys 2012;84:e351-6. [Crossref] [PubMed]
- Cella L, Liuzzi R, Conson M, et al. Development of multivariate NTCP models for radiation-induced hypothyroidism: a comparative analysis. Radiat Oncol 2012;7:224. [Crossref] [PubMed]
- Rønjom MF, Brink C, Bentzen SM, et al. External validation of a normal tissue complication probability model for radiation-induced hypothyroidism in an independent cohort. Acta Oncol 2015;54:1301-9. [Crossref] [PubMed]
- Amdur RJ, Liu C, Li J, et al. Matching intensity-modulated radiation therapy to an anterior low neck field. Int J Radiat Oncol Biol Phys 2007;69:S46-8. [Crossref] [PubMed]
- Ho FC, Lu JJ. Management of the node-negative neck in early-stage nasopharyngeal carcinoma. Available online: https://doi.org/
10.2217/ebo.13.612 - Ho FC, Tham IW, Earnest A, et al. Patterns of regional lymph node metastasis of nasopharyngeal carcinoma: a meta-analysis of clinical evidence. BMC cancer 2012;12:98. [Crossref] [PubMed]
- Langendijk JA, Lambin P, De Ruysscher D, et al. Selection of patients for radiotherapy with protons aiming at reduction of side effects: the model-based approach. Radiother Oncol 2013;107:267-73. [Crossref] [PubMed]
- Widder J, van der Schaaf A, Lambin P, et al. The Quest for Evidence for Proton Therapy: Model-Based Approach and Precision Medicine. Int J Radiat Oncol Biol Phys 2016;95:30-6. [Crossref] [PubMed]
- Rønjom MF, Brink C, Bentzen SM, et al. Hypothyroidism after primary radiotherapy for head and neck squamous cell carcinoma: normal tissue complication probability modeling with latent time correction. Radiother Oncol 2013;109:317-22. [Crossref] [PubMed]
- Beetz I, Schilstra C, van Luijk P, et al. External validation of three dimensional conformal radiotherapy based NTCP models for patient-rated xerostomia and sticky saliva among patients treated with intensity modulated radiotherapy. Radiother Oncol 2012;105:94-100. [Crossref] [PubMed]
Cite this article as: Prayongrat A, Lertbutsayanukul C. Hypothyroidism after radiotherapy for nasopharyngeal carcinoma. Ann Nasopharynx Cancer 2020;4:3.