
Combination of precision radiotherapy with chemotherapy and immunotherapy in non-recurrent/metastatic nasopharyngeal carcinoma
Introduction
Nasopharyngeal carcinoma (NPC) is a special type of head and neck cancer because of its unique epidemiology and association with Epstein-Barr virus (EBV) infection. NPC has a unique geographical distribution, being mainly prevalent in Southeast Asia. According to the International Agency for Research on Cancer, the incidence of NPC in 2018 was 129,079 worldwide, of which 60,558 (46.9%) cases were in China, and 34,639 (26.8%) cases occurred in the rest of Southeast Asia (1). Correspondingly, the age-standardized incidence of NPC in 2018 ranged from 2.0 to 6.6 in southeast Asia, while it ranged from 0.21 to 0.51 in non-endemic North America (1). In addition to environmental factors, ethnic and genetic factors also play an important role in the pathogenesis of NPC, which has been validated in second-generation immigrants from endemic regions (2).
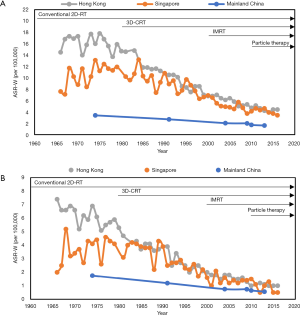
Radiotherapy is the backbone of NPC treatment. Over recent decades, the precision of radiotherapy techniques has increased rapidly, from the conventional two-dimensional radiotherapy (2D-RT) to the three-dimensional radiotherapy (3D-RT), which includes three-dimensional conformal radiotherapy (3D-CRT, intensity-modulated radiotherapy (IMRT), and particle beam therapy (such as proton therapy and carbon-ion therapy). The progress of precision radiotherapy has brought appreciably better survival outcomes, with obviously decreased mortality rate observed in three NPC endemic regions (Figure 1) (3-8). From 1974 to 2013, the mortality rate of male patients with NPC decreases by 73.8% in Hong Kong, 64.9% in Singapore, and 65.7% in Mainland China. However, radiotherapy alone is not a high intensity treatment option for locoregionally advanced NPC (LANPC). A suitable way to achieve effective management of LANPC is to add chemotherapy to radiotherapy, such as concurrent chemoradiotherapy, which is regarded as the standard choice according to the National Comprehensive Cancer Network (NCCN) clinical guidelines. Besides, the emerging immunotherapy promises to be alternative to the current standard care in NPC.
The most suitable schedule and regimen of combination therapy comprising chemo-/immunotherapy based on precision radiotherapy are still under extensive investigation, and there is controversy surrounding the limited and heterogenous trial results. Therefore, this comprehensive review discusses the efficacy and safety of various combinations of precision radiotherapy with chemotherapy and immunotherapy in non-recurrent/metastatic NPC, with the aim of providing an insight into related ongoing and future clinical trials.
Precision radiotherapy
Radiotherapy has long been the backbone of the treatment modality for non-recurrent/metastatic NPC since 1965, because of its unique biological behaviour of high radiosensitivity and deep anatomical position. In the 1920s, only a few patients with NPC receiving radiotherapy alone survived for more than 3 years (9). Longer survival has been achieved since the development of the machine from kilo-voltage to mega-voltage, which yielded a 5-year overall survival (OS) of 25% by 1965 (10). Since then, although still in the era of conventional 2D-RT, the prognosis of patients with NPC gradually improved. Retrospective analysis of 5,037 patients with NPC treated between 1976 and 1985 in Hong Kong and 378 patients treated between 1954 and 1992 in M.D. Anderson Cancer Centre reported a similar 5-year OS of over 50% and a local control rate of 60–70% (11,12). Another study of 2,687 patients with NPC treated between 1996 and 2000 showed a 5-year OS of 75% and a local failure-free rate of 85% (13).
The nasopharynx lies in a special anatomical position adjacent to many critical structures, including salivary glands, ear, pharyngeal muscle, mandible, oral cavity, spinal cord, brain, and brainstem; therefore, unavoidable radiation to these structures results in life-related toxicities (e.g., xerostomia, mucositis, hearing loss, dysphagia, jaw osteonecrosis, or brain injury), which significantly impaired patients’ quality of life. Considering that it is impossible to avoid casting the beam through the structures, highly precise methods that deliver most of the beam to the tumor while sparing more normal tissues are required. From the 1990s, along with the development of computer science, imaging techniques [e.g., computed tomography (CT), magnetic resonance imaging (MRI), positron emission tomography-CT (PET-CT)], and radiotherapy machines themselves, major radiotherapeutic techniques have gradually advanced from 2D-RT to the more precise 3D-RT. A series of 3D-RT techniques have been adopted to treat NPC over the past two decades, including 3D-CRT, IMRT, stereotactic radiotherapy, etc. In 3D-CRT, the development of CT enables the delineation of tumors in three dimensions, as opposed to the “flat” image from X-ray, and the advent of multileaf collimators has helped to arrange beams to optimally fit the outlines of the tumors at various angles. More precise than 3D-RCT, IMRT deliver a non-uniform fluence to the tumor, by dividing the beam into multiple “beamlets” with different intensities. Many publications have reported significantly improved therapeutic effects of IMRT compared with those of 2D-RT (14-21). The local control rate (LCR) has increased from 44–68% to 75–95% for LANPC (20,22-28). A meta-analysis including 3,570 participants in 8 studies showed that IMRT resulted in a better 5-year OS [odds ratio (OR) 1.51, 95% confidence interval (CI): 1.23–1.87] and 5-year LCR compared with those achieved by 2D-RT or 3D-CRT (29). Besides, a prospective randomized controlled trial showed that IMRT resulted in an improved 5-year OS (79.6% vs. 67.1%) and LCR (90.5% vs. 84.7%) compared with that of 2D-RT (30). In addition to the survival benefit, IMRT improves quality of life by sparing more organs at risk (OARs) and reducing radiation-induced toxicities. A randomized clinical trial revealed that IMRT led to a significantly lower incidence of xerostomia than 2D-RT (39.3% vs. 82.1%) and a better preservation of parotid function (31). Another trial demonstrated a significantly lower incidence of both acute and late radiation-induced toxicities from IMRT (30). Based on these evidences, IMRT has become the most widely used radiotherapy technique in current clinical practice.
However, the intratreatment and intertreatment variation of the tumor poses a great threat to the precision of radiotherapy. Intratreatment variation results mainly from movement, while intertreatment variation is mainly from tumor shrinkage, soft-tissue change, and weight loss. Considering the very steep dose fall-off at the margin between the tumor and normal tissue in IMRT, these variations could lead to missing the target and an overdosage in the OARs. Image-guided radiotherapy (IGRT) was introduced to cope with this issue, which uses imaging within the treatment room to change the position of the patient to match with the primary CT-simulation or change the planning dose. A prospective study of 197 patients with NPC reported a survival superiority in 5-year OS of IGRT over non-IGRT (91.8% vs. 74.3%) (32), while another study failed to detect a significant difference of the dose delivered to parotid glands between the IGRT and non-IGRT groups (33). Tomotherapy, an advanced form of IMRT that combines IGRT with a helical delivery pattern, is believed to have a better dosimetric distribution. A prospective phase II clinical trial comparing tomotherapy with IMRT in patients with NPC is still underway (NCT03588403). More recently, adaptive radiotherapy, an emerging concept that involves replanning during the treatment by changing either the dosage or outlines, is a promising technique to deal with these variations. Studies have demonstrated a survival benefit of adaptive radiotherapy in head and neck cancer, including NPC (34), and a prospective non-inferiority trial of the use of adaptive radiotherapy for head and neck cancer undergoing radiotherapy is proposed (NCT03096808); however, more studies on the details of implementation and identification of the benefits of adaptive radiotherapy are warranted.
Over the past decade, emerging particle radiotherapy techniques, including proton therapy and carbon ion radiotherapy, have made great progress and attracted increased interest in their application to treat NPC, as an alternative to the traditional photon-based radiotherapy mentioned above. Particle beams establish a unique distribution of dose in depth, known as the “Brag Peak”, creating a much sharper dose fall-off by releasing most of the energy over a short range of depth, depending on the initial energy level, and releasing only a little outside the peak, which provides a much more precisely controlled dose distribution. The dosimetric advantage of intensity-modulated proton therapy brings a significant improvement in tumor conformation and a reduction in both the mean dose to the OARs and the relevant radiation-induced toxicities, compared with those of IMRT (35-42). Favorable clinical outcomes of proton therapy have been reported in many publications. Preliminary results of a phase II trial of proton therapy with chemotherapy for NPC in Massachusetts General Hospital (NCT00592501) showed a 2-year LCR, OS, and disease-specific survival of 100%, 100%, and 90%, respectively (43). A similar result was reported in MD Anderson Cancer Center, with a 2-year LCR of 100% and OS of 88.9% (35). Intensity-modulated carbon-ion radiation therapy also shows a dosimetric advantage over IMRT in sparing more critical OARs (44). Studies of carbon ion radiotherapy are mainly limited to high-risk or recurrent NPC, mainly because of its high cost and limited availability. A study including 24 patients with high-risk NPC receiving bimodal treatment comprising IMRT plus carbon ion radiotherapy reported a 2-year LCR and OS of 95% and 100%, respectively (45). The first prospective trial evaluating the efficacy and safety of intensity-modulated carbon-ion radiation therapy in locoregional recurrent NPC has reported preliminary results. It showed an increase of 1-year OS from the historical 82% to 95% for intensity-modulated carbon-ion radiation therapy (46,47), which is in line with the value (98.1%) published in a retrospective study (48). Results of a phase I/II trial evaluating carbon ion radiation therapy for locally recurrent NPC is due imminently (NCT02795195). Currently, the availability of particle radiotherapy is limited, mainly because of the high cost and large size of the machines. There are only 5 countries and 13 centers that provide carbon ion radiotherapy and there are fewer than 100 proton therapy centers worldwide (49), which has also limited the evidence supporting particle therapy in NPC. No randomized trials evaluating particle therapy in NPC are available yet; therefore, further study of particle therapy is expected, especially trials with both a larger sample size and a randomized setting.
Precise dose planning is crucial to precision radiotherapy, in addition to the radiation techniques. Significant inter-observer variation was observed in manual contouring for all OARs of NPC, which affected dosimetric parameters significantly (50). To reduce the intra- and inter-observer variation, the idea of an automated artificial intelligence contouring system based on deep learning, especially convolutional neural networks, has been proposed against the background of booming artificial intelligence techniques. Findings showed that the artificial intelligence contouring system to automate delineation of the primary gross tumor volume could significantly improve accuracy and reduce variation and contouring time (51).
Combining chemotherapy with precision radiotherapy
With rapidly increasing precision, very satisfactory clinical outcomes have been achieved using radiotherapy alone for patients with early-stage NPC in the era of IMRT. Radiotherapy alone is recommended as the first choice for patients with T1N0M0 NPC (52). For patients with NPC other than stage I, radiotherapy alone might be insufficient; thus, at least one type of systematic therapy is recommended to be added to radiotherapy for these patients, according to the 2020 NCCN clinical guidelines (52). However, consensus has yet to be achieved for the management of stage II non-metastatic NPC. The 10-year survival outcomes of a phase III randomized clinical trial indicated that concurrent chemoradiotherapy (CCRT) significantly improves OS, progression-free survival (PFS), and distant metastasis-free survival (DMFS), compared with those achieved by 2D-RT alone (53), while a meta-analysis showed that IMRT alone achieved similar survival outcomes compared with those of CCRT in stage II NPC (54). Another retrospective study demonstrated that CCRT only shows a survival advantage over 2D-RT, but not IMRT (55). A phase III multicenter randomized clinical trial (NCT02633202) focusing on this issue is underway. In this study, a total of 338 patients with stage T1–2N1M0/T2–3N0M0 NPC were randomly assigned into an IMRT group or a CCRT group, which might provide a clearer answer to whether chemotherapy is necessary for stage II NPC in the era of IMRT.
Concurrent chemoradiotherapy
Definitive CCRT is recognized as the standard treatment modality for LANPC, based on the evidence derived from many clinical trials; however, these were mainly performed in the era of 2D-RT (Table 1) (56,59,63,64). A meta-analysis conducted by Blanchard and colleagues in 2015 revealed that an OS benefit was only observed in CCRT [hazard ratio (HR) 0.65, 95% CI: 0.56–0.76] or CCRT plus adjuvant chemotherapy (AC; HR 0.65, 95% CI: 0.56–0.76) in comparison with radiotherapy alone (65). Meanwhile, another meta-analysis demonstrated a significantly improved 5-year OS (relative risk 0.64, 95% CI: 0.45–0.91) and overall response rate (0.53; 95% CI: 0.43–0.66) in CCRT versus IMRT alone (66). Besides, an ongoing multicenter clinical trial might provide more evidence of the superiority of CCRT over radiotherapy alone (NCT01817023).
Table 1
| Reference | Experimental chemotherapy | Control chemotherapy | Inclusion |
Sample size | Overall survival | Other survival outcome measurements | |||
|---|---|---|---|---|---|---|---|---|---|
| Experimental vs. control | HR (95% CI); P value | Experimental vs. control | HR (95% CI); P value | ||||||
| Concurrent chemoradiotherapy vs. radiotherapy alone | |||||||||
PFS, progress-free survival; FFS, failure-free survival; DMFS, distant metastasis-free survival; CI, confidence interval; HR, hazard ratio; NR, not reported; q1/3/4wks, every 1/3/4 weeks; d1–4: day 1 to day 4.
Cisplatin is regarded as the classic chemotherapy regimen for NPC in the CCRT regimen, and is also the only drug that the 2020 NCCN clinical guidelines recommended for CCRT (52,57,63). The efficacy of drugs other than cisplatin, including other platinum-based drugs like carboplatin, oxaliplatin, lobaplatin, or nedaplatin (62,67-69), and non-platinum drugs like uracil plus tegafur, docetaxel, or 5-fluorouracil plus hydroxyurea (70-72), has been widely explored. Some studies showed that the non-platinum regimen has a comparable therapeutic effect to cisplatin, which might offer more alternatives for NPC. A recent retrospective cohort study revealed that CCRT based on non-platinum regimens was inferior in terms of OS and disease-free survival (DFS) to the platinum-based CCRT, although the differences were not significant (73). Regarding the dose of cisplatin in clinical practice, either 80–100 mg/m2 every 3 weeks or 40 mg/m2 once a week is acceptable (8), and a cumulative cisplatin dose of 230–270 mg/m2 is recommended for patients with LANPC (74).
Adjuvant chemotherapy (AC)
Although CCRT has been proven to be highly effective in locoregional control, distant metastasis is still an unresolved problem that requires additional cycles of chemotherapy to strengthen treatment intensity. However, no evidence supporting AC alone has been reported yet. Several studies reported that AC following radiotherapy could not bring survival benefits, but induced increased toxicities (58,60,65,75).
More attention has been paid to the combination of AC and CCRT. In 1998, the landmark American Intergroup-0099 Study (INT-0099) demonstrated a significant improvement in 3-year OS and PFS in concurrent-adjuvant chemoradiotherapy compared with that of radiotherapy alone (63). However, there are two major flaws in the INT-0099 study. First, as radiotherapy alone was set as the control group in comparison with CCRT plus AC, it is difficult to definitively determine which one of the two chemotherapy schedules, or both of them, was the real and effective modality. Second, the INT-0099 trial was conducted in North America, where the major pathological type of NPC is World Health Organization type I, which is different from the endemic regions, where type II/III dominate. Therefore, the results of the INT-0099 study might be inapplicable to endemic regions. Since then, the efficacy of the INT-0099 regimen (cisplatin plus 5-fluorouracil as AC) has been validated in three endemic regions, including Hong Kong (NPC-9901 and NPC-9902 trials), Singapore (SQNP01 trial), and Mainland China (Table 2) (58,76-80). A combined analysis of NPC-9901 and NPC-9902 revealed that the CCRT phase and AC phase had a significant impact on locoregional failure-free survival and distant failure-free survival, respectively (83). However, consensus on whether AC following CCRT will achieve more survival benefits compared with those of CCRT alone, without increasing toxicity, has yet to be achieved. A robust phase III trial showed that CCRT followed by AC (cisplatin and 5-fluorouracil) failed to improve FFS, OS, DMFS, and LFFS compared with those achieved by CCRT alone in LANPC (Table 3) (60,61). Blanchard’s meta-analysis also presented a similar result (65). It seems that applying concurrent-adjuvant chemoradiotherapy to patients with LANPC at a high risk of treatment failure might provide a survival benefit. A cohort study generating a risk stratification using age, T/N classification, and serum albumin level suggested that CCRT followed by AC (cisplatin and 5-fluorouracil, cisplatin and docetaxel, or cisplatin, 5-fluorouracil and docetaxel) could improve OS compared with the use of CCRT alone in high-risk LANPC (94). Besides, the post-treatment plasma EBV DNA level has become the most promising biomarker for risk stratification (95,96). A retrospective cohort defined high-risk patients as those with persistently detectable plasma EBV DNA 1 week after radiotherapy, and treated them with oral tegafur-uracil, with or without intravenous mitomycin-C, epirubicin, and cisplatin, while the control group was only placed under surveillance. This study showed a significant improvement of 5-year OS (71.6% vs. 28.7%; HR 0.27, 95% CI: 0.17–0.55) and a reduction in distant failure (97). However, in the phase III NPC-0502 trial, high-risk patients were defined as carrying detectable plasma EBV DNA at 6 to 8 weeks after radiotherapy and received AC comprising gemcitabine plus cisplatin (GP), while the rest of the patients were only placed under surveillance. No improvement in 5-year recurrence-free survival (49% vs. 55%; HR 1.09, 95% CI: 0.63–1.89) or OS (64% vs. 68%; HR 1.09, 95% CI: 0.56–2.11) was observed for AC compared with observation (98). The ongoing phase II/III NRG-HN001 trial (NCT02135042) defines high-risk patients as those with detectable plasma EBV DNA 1 week after IMRT. It is attempting to evaluate whether using adjuvant gemcitabine and paclitaxel could yield better survival outcomes than the standard regimen of cisplatin and 5-fluorouracil, which might provide further support for the application of AC. A recent new risk stratification model integrating the TNM staging system and post-treatment plasma EBV DNA showed improved effectiveness to screen patients with NPC, which could aid the selection of the AC beneficiaries in clinical practice (99).
Table 2
| Reference | Experimental chemotherapy | Inclusion |
Sample size | Overall survival | Other survival outcome measurements | |||
|---|---|---|---|---|---|---|---|---|
| Experimental vs. control | HR (95% CI); P value | Experimental vs. control | HR (95% CI); P value | |||||
| Concurrent chemoradiotherapy plus adjuvant chemotherapy vs. radiotherapy alone | ||||||||
| Al-Sarraf et al. (63) | Concurrent: cisplatin 100 mg/m2 d1, q3wks × 3; Adjuvant: cisplatin 80 mg/m2 d1, fluorouracil 1,000 mg/m2 d1–4, q4wks × 3 | 1989–1995 | 147 | 3-year: 78% vs. 47% | 2.50 (1.29–4.84); P=0.005 | 3-year PFS: 69% vs. 24% | 4.34 (2.47–7.69); P<0.001 | |
| Wee et al. (76) | Concurrent: cisplatin 25 mg/m2 d1–4, q3wks × 3; Adjuvant: cisplatin 20 mg/m2 d1–4, fluorouracil 1,000 mg/m2 d1–4, q4wks × 3 | 1997–2003 | 221 | 3-year: 80% vs. 65% | 0.51 (0.31–0.81); P=0.0061 | 3-year DFS: 72% vs. 53% | 0.57 (0.4–0.9); P=0.093 | |
| Lee et al. (77,78) | Concurrent: cisplatin 100 mg/m2 d1, q3wks × 3; Adjuvant: cisplatin 80 mg/m2 d1, fluorouracil 1,000 mg/m2 d1–4, q4wks × 3 | 1999–2004 | 348 | 10-year: 62% vs. 49% | 0.74 (0.56–0.997); P=0.047 | 10-year PFS: 56% vs. 42% | 0.68 (0.51–0.90); P=0.006 | |
| Lee et al. (79) | Concurrent: cisplatin 100 mg/m2 d1, q3wks × 3; Adjuvant: cisplatin 80 mg/m2 d1, fluorouracil 1,000 mg/m2 d1–4, q4wks × 3 | 1999–2004 | 189 | 5-year: 78% vs. 66% | 0.76 (0.38–1.54); P=0.45 | 5-year PFS: 60% vs. 62% | 0.98 (0.53–1.81); P=0.93 | |
| Chen et al. (80) | Concurrent: cisplatin 40 mg/m2 d1, q3wks × 7; Adjuvant: cisplatin 80 mg/m2 d1, fluorouracil 800 mg/m2 d1–5, q4wks × 3 | 2002–2005 | 316 | 5-year: 72% vs. 62% | 0.69 (0.48–0.99); P=0.043 | 5-year PFS: 68% vs. 57% | 0.65 (0.46–0.92); P=0.015 | |
| Induction chemotherapy followed by radiotherapy vs. radiotherapy alone | ||||||||
| Ma et al. (82) | Induction: bleomycin 10 mg/m2 d1, d5, cisplatin 100 mg/m2 d1, fluorouracil 800 mg/m2 d1–5, q3wks × 2–3 | 1993–1994 | 456 | 5-year: 63% vs. 56% | NR; P=0.11 | 5-year RFS: 82% vs. 74% | NR; P=0.05 | |
PFS, progress-free survival; DFS, disease-free survival; FFS, failure-free survival; RFS, recurrence-free survival; DMFS, distant metastasis-free survival; CI, confidence interval; HR, hazard ratio; NR, not reported; q3/4wks, every 3/4 weeks.
Table 3
| Reference | Experimental chemotherapy | Control chemotherapy | Inclusion period | Sample size | Overall survival | Other survival outcome measurements | ||
|---|---|---|---|---|---|---|---|---|
| Experimental vs. control | HR (95% CI); P value | Experimental vs. control | HR (95% CI); P value | |||||
| Induction chemotherapy plus concurrent chemoradiotherapy vs. concurrent chemoradiotherapy | ||||||||
| Tan et al. (84) | Induction: gemcitabine 1,000 mg/m2 d1, d8, carboplatin AUC = 2.5 d1, d8, paclitaxel 70 mg/m2 d1, d8, q3wks × 3; Concurrent: cisplatin 40 mg/m2 d1, q1wk × 8 | Concurrent: cisplatin 40 mg/m2 d1, q1wk × 8 | 2004–2012 | 172 | 3-year: 94% vs. 92% | 1.05 (0–2.19); P=0.49 | 3-year DFS: 75% vs. 67% | 0.77 (0.44–1.35);P=0.36 |
| Li et al. (85,86) | Induction: docetaxel 60 mg/m2 d1, cisplatin 60 mg/m2 d1, fluorouracil 600 mg/m2 d1–5, q3wks × 3; Concurrent: cisplatin 100 mg/m2 d1, q3wks × 3 | Concurrent: cisplatin 100 mg/m2 d1, q3wks × 3 | 2011–2013 | 480 | 5-year: 86% vs. 78% | 0.65 (0.43–0.98); P=0.042 | 5-year FFS: 77% vs. 66% | 0.65 (0.43–0.98); P=0.019 |
| Yang et al. (87,88) | Induction: cisplatin 80 mg/m2 d1, fluorouracil 800 mg/m2 d1–5, q3wks × 2; Concurrent: cisplatin 80 mg/m2 d1, q3wks × 3 | Concurrent: cisplatin 80 mg/m2 d1, q3wks × 3 | 2008–2015 | 476 | 5-year: 80.8% vs. 76.8% | 0.69 (0.49–0.98); P=0.040 | 5-year DFS: 73.4% vs. 63.1% | 0.66 (0.48–089); P=0.007 |
| Hong et al. (89) | Induction: mitomycin 8 mg/m2 d1, epirubicin 60 mg/m2 d1, cisplatin 60 mg/m2 d1, fluorouracil 450 mg/m2 d8, leucovorin 30 mg/m2 d8; Concurrent: cisplatin 30 mg/m2 d1, q1wk | Concurrent: cisplatin 30 mg/m2 d1, q1wk | 2003–2009 | 479 | 5-year: 72% vs. 68% | 0.92 (0.67–1.27); P=0.62 | 5-year DFS: 61% vs. 50% | 0.74 (0.57–0.97); P=0.026 |
| Frikha et al. (90) | Induction: docetaxel 75 mg/m2 d1, cisplatin 75 mg/m2 d1, fluorouracil 750 mg/m2 d1–5, q3wks × 3; Concurrent: cisplatin 40 mg/m2 d1, q1wk × 7 | Concurrent: cisplatin 40 mg/m2 d1, q1wk × 7 | 2009–2012 | 81 | 3-year: 86% vs. 69% | 0.40 (0.15–1.04); P=0.059 | 3-year PFS: 74% vs. 57% | 0.44 (0.20–0.97); P=0.042 |
| Zhang et al. (91) | Induction: gemcitabine 1,000 mg/m2 d1, d8, cisplatin 80 mg/m2 d1, q3wks × 3; Concurrent: cisplatin 100 mg/m2 d1, q3wks × 3 | Concurrent: cisplatin 100 mg/m2 d1; q3wks × 3 | 2013–2016 | 480 | 3-year: 94.6% vs. 90.3% | 0.43 (0.24–0.77); P=NR | 3-year RFS: 85.3% vs. 76.5% | 0.51 (0.34–0.77); P=0.002 |
| Concurrent chemoradiotherapy plus adjuvant chemotherapy vs. concurrent chemoradiotherapy | ||||||||
| Chen et al. (60,61) | Concurrent: cisplatin 40 mg/m2 d1; Adjuvant: cisplatin 80 mg/m2 d1, fluorouracil 800 mg/m2 d1–5, q4wks × 3 | Concurrent: cisplatin 40 mg/m2 d1 | 2006–2010 | 251 | 5-year: 83% vs. 80% | 0.86 (0.57–1.22); P=0.35 | 5-year FFS: 75% vs. 71% | 0.88 (0.64–1.22); P=0.45 |
| Induction chemotherapy followed by radiotherapy vs. concurrent chemoradiotherapy | ||||||||
| Xu et al. (92,93) | Induction: cisplatin 90 mg/m2, fluorouracil 1,500 mg/m2, q3wks × 2; Adjuvant: cisplatin 90 mg/m2, fluorouracil 1,500 mg/m2, q3wks ×4 | Concurrent: cisplatin 90 mg/m2, fluorouracil 1,500 mg/m2, q3wks ×2; Adjuvant: cisplatin 90 mg/m2, fluorouracil 1,500 mg/m2, q3wks ×4 | – | 338 | 5-year: 79% vs. 76% | 0.84 (0.53–1.33); P=0.47 | 5-year DMFS: 77% vs. 87% | 0.59 (0.35–1.00); P=0.05 |
PFS, progress–free survival; DFS, disease–free survival; FFS, failure–free survival; RFS, recurrence–free survival; DMFS, distant metastasis–free survival; CI, confidence interval; HR, hazard ratio; NR, not reported; q3/4wks, every 3/4 weeks.
Considering the controversies identified to date, it is difficult to draw a definitive conclusion regarding the utility of AC. On the one hand, marked between-study heterogeneity exists in study design, AC regimens and schedules, risk stratification methods, and even the timing of detection of post-treatment plasma EBV DNA levels. On the other hand, patients showed generally low compliance with the completion rate of whole-course AC, ranging from 50% to 76%, which was caused by poor fidelity to treatment resulting from severe toxicities (25,63,76,78-80). This greatly impaired the practicability and generalizability of the study results. The introduction of metronomic chemotherapy, a new method of delivering chemotherapeutic drugs in a continuous and dose-dense way, might help solve the latter problem by reducing toxicity and improving compliance (100). A retrospective analysis reported that the metronomic use of tegafur-uracil as AC significantly improved the 5-year DFS (91.89% vs. 57.58%), without compromising safety, compared with that of observation after radiotherapy (101). A prospective phase II trial came to a similar conclusion using metronomic delivery of capecitabine as AC (102). Three ongoing phase III randomized controlled trials evaluating the efficacy and toxicity of capecitabine following CCRT in patients with LANPC have attracted considerable attention, as one of them (NCT02958111) is delivered in a metronomic way (1,300 mg/m2 per day for 1 year) and the other two trials (NCT02973386 and NCT02143388) use a traditional method of treatment delivery. The results of these trials will provide more insight into the efficacy of the adjuvant regimen using single-capecitabine and the impact of metronomic AC on the prognosis of patients with NPC.
Induction chemotherapy (IC)
IC is thought to be better tolerated than AC (103,104). The upfront use of chemotherapeutic drugs is not only more effective in reducing micrometastases, but also provides a wider safety zone and flexibility for radiotherapy planning by shrinking the tumors before radiotherapy (105). However, whether the combination of IC and radiotherapy is superior to radiotherapy alone remains controversial, because inconsistent results were reported by a series of studies comparing IC followed by radiotherapy with radiotherapy alone in LANPC. In 1996, a phase II trial reported that the combination of IC (bleomycin, epirubicin, and cisplatin) and radiotherapy improved DFS compared with that achieved by radiotherapy alone (106). Similarly, a pooled analysis of two phase III trials showed that adding cisplatin-based IC to radiotherapy led to a significantly improved disease-specific survival in LANPC (Table 2) (81,82,103). By contrast, a cohort study showed that IC (cisplatin and 5-fluorouracil) followed by radiotherapy significantly improved 5-year OS and DFS (107). However, a clinical trial adopting the same IC regimen did not find any significant differences in 5-year OS and DFS (108). Based on the studies conducted using 2D-RT, a meta-analysis concluded that IC could decrease the risk of recurrence and metastasis, but not improve OS and DFS, compared with that achieved by radiotherapy alone (109). However, the insignificant survival superiority of IC followed by radiotherapy was not validated in the era of IMRT (110). A retrospective study showed that patients with stage II NPC could benefit from adding IC (cisplatin plus docetaxel or 5-fluorouracil) to IMRT (111). Considering that CCRT is now the mainstay treatment of LANPC, it is more meaningful to perform a direct comparison of IC followed by radiotherapy with CCRT. However, past studies indicated that the differences in survival were not significant between the two treatment modalities, regardless of what kind of IC drugs were used (Table 3) (92,93,112-115). An ongoing phase III randomized controlled trial (NCT02434614) has the potential to deliver critical medical information concerning whether concurrent chemotherapy could be omitted when IC is combined with IMRT.
IC followed by CCRT is a more promising strategy to treat NPC, which has been studied in many randomized controlled trials (Table 3). A phase II clinical trial showed that IC (cisplatin and docetaxel) followed by CCRT significantly improved 3-year OS compared with that achieved by CCRT alone (116), while the other two phase II trials using different IC regimens (cisplatin-epirubicin and carboplatin-gemcitabine-paclitaxel) failed to detect a survival difference (84,117). The conflicting results might result from the small sample size and the natural flaw of the phase II trial design. Two multicenter phase III clinical trials, the GZ2011 trial adopting the IC regimen of docetaxel, cisplatin, and 5-fluorouracil (TPF); and the GZ2008 trial using cisplatin and 5-fluorouracil, reported a significant survival benefit in terms of PFS of IC followed by CCRT versus CCRT alone in LANPC (85,87). In addition, the GORTEC 2006 phase III trial showed that the addition of the TPF IC regimen significantly improved 3-year PFS (90). The long-term results of the GZ2011 and GZ2008 trials further validated the survival advantage of IC followed by CCRT (86,88). Meanwhile, improved 5-year DFS achieved by additional IC using mitomycin, epirubicin, cisplatin, 5-fluorouracil, and leucovorin was observed in the phase III TCOG 1303 study (89). A pooled analysis of four randomized controlled trials found that IC followed by CCRT improved OS and PFS, and reduced locoregional and distant failures in LANPC (118). These results have been validated in a recent meta-analysis (119). Considering the survival benefits and good tolerance of IC, the recommendation evidence of IC followed by CCRT has been upgraded from level 3 to 2A in the NCCN clinical guidelines since 2018, which is the same level as CCRT followed by AC (120), indicating that IC will play an increasingly important role in LANPC treatment.
However, the optimal IC regimen has yet to be established. The effectiveness and toxicity of the TPF IC regimen have been validated in two phase III trials (121,122). A pooled analysis demonstrated no significant differences in survival outcomes among the TPF, docetaxel-cisplatin, and cisplatin-5-fluorouracil IC regimens; however, only the TPF regimen significantly improved OS and PFS compared with that achieved by the group without IC (118). A meta-analysis reported that TPF IC followed by CCRT led to better survival with tolerable toxicities compared with that of CCRT alone or double-drug-based IC plus CCRT (123). Recently, the GP regimen, which had proven its efficacy in recurrent or metastatic NPC (124), has been studied in LANPC as an alternative to the TPF regimen. A multicenter phase III randomized controlled trial reported that GP IC followed by CCRT improved 3-year recurrence-free survival (94.6% vs. 90.3%; HR 0.43; 95% CI: 0.24–0.77) and OS (85.3% vs. 76.5%; HR 0.51; 95% CI: 0.34–0.77) compared with that of CCRT alone in LANPC, and the high compliance of 96.7% supported its good toleration (91). A retrospective cohort study showed that the GP and TPF regimens achieved similar efficacy; however, the GP regimen is associated with increased hepatotoxicity (125). Further prospective studies or clinical trials comparing the roles of TPF, GP, and other regimens in IC are warranted. An ongoing phase III clinical trial (NCT03840421) comparing GP with cisplatin-5-fluorouracil as the IC regimen followed by CCRT in LANPC might provide more evidence.
Combining immunotherapy with radiotherapy
The unique characteristics of NPC, including its association with EBV infection, abundant tumor-infiltrating lymphocytes (TIL) in NPC tissues, and high expression of programmed cell death-ligand 1 (PD-L1) of up to 90%, make immunotherapy a promising treatment modality for NPC (126-129). Generally, anti-cancer immunotherapy consists of cancer vaccination, monoclonal antibodies, immune checkpoint inhibitors, adoptive T-cell therapy, and cytokines. Among them, vaccination and adoptive T-cell therapy targeting EBV-specific antigens were tested in patients with recurrent or metastatic NPC, and showed potential clinical efficacy (130-133); however, the combination of these therapeutic strategies with radiotherapy has not been explored.
Considering the high expression of PD-L1 and abundant TIL in NPC, applying immune checkpoint inhibitors, such as programmed cell death-1 (PD-1) and PD-L1 monoclonal antibodies, to LANPC is very appealing, especially with the emerging evidence of the synergistic radiotherapy-immunity interaction. On the one hand, experiments showed that radiotherapy could alter the immune context and microenvironment of the tumor to trigger an anti-tumor immune response. Two preclinical studies revealed an upregulation of PD-L1 levels in the tumors of mice after radiotherapy (134,135). The increased level of immunosuppressive regulatory T cells (Tregs) were found within the tumor after radiotherapy in vivo (136). Thus, the pro-immune effect induced by radiotherapy might not emerge without the help of immunotherapy. On the other hand, the role of PD-L1 blockade as a radiosensitizer has been observed both in vivo and in vitro (134,137), which might improve the efficacy of radiotherapy in patients with radioresistant NPC and reduce the irradiation dosage of radiotherapy to preserve more OARs. KEYNOTE-028 is a phase Ib trial evaluating the efficacy of PD-1 blockade using pembrolizumab. The results showed that pembrolizumab had satisfactory outcomes in 27 patients with PD-L1-positive, treatment-naïve, locally advanced or metastatic NPC, with an objective response rate (ORR) of 26%, a 1-year OS of 63%, a 1-year PFS of 33%, and manageable toxicity (138). A phase II trial evaluating nivolumab in 44 patients with previously treated recurrent or metastatic NPC demonstrated comparable results (ORR 20%, 1-year OS 59%, 1-year PFS 19%) (139). In addition, the combination of PD-1 blockade using camrelizumab with GP chemotherapy sharply increased the ORR from 34% to 91% in recurrent or metastatic NPC (140). These results support the extension of immune checkpoint inhibitors into combination with radiotherapy or chemoradiotherapy. Several ongoing randomized controlled trials evaluating the efficacy of combination therapy comprising immune checkpoint inhibitors with IMRT or CCRT are highly anticipated (Table 4). A phase II trial (NCT03383094) comparing concurrent pembrolizumab and radiotherapy with CCRT in p16-positive locoregionally advanced head and neck squamous cell carcinoma including NPC is ongoing. A single-arm multicenter phase II clinical trial (NCT03984357) is evaluating the efficacy and safety of whole-course concurrent and adjuvant nivolumab combined with IC followed by radiotherapy alone in LANPC. This is the first attempt to develop a de-intensification therapy by sparing the cisplatin-based concurrent chemotherapy and adopting PD-1 blockade based on IC followed by IMRT alone in LANPC. Meanwhile, two phase III clinical trials conducted by Ma et al. will investigate the value of concurrent PD-1 blockade using sintilimab (NCT03700476) and adjuvant PD-1 blockade using camrelizumab (NCT03427827) when added to standard chemoradiotherapy in LANPC. Given the additional toxicities induced by immunotherapy, selection of the beneficiaries of the combination of immunotherapy and radiotherapy is expected to be important; however, no effective biomarkers have yet been identified.
Table 4
| Trials | Phase | Sample size | Multicenter | Key eligibility criteria | Experimental regimen | Control regimen |
|---|---|---|---|---|---|---|
| Ma et al. (NCT03984357) | II | 146 | Yes | Stage III–IVA (except T3–4N0 and T3N1) | Induction: gemcitabine 1,000 mg/m2 d1, d8; cisplatin 80 mg/m2 d1; nivolumab 360 mg d1; q3wks × 3 | NA |
| Concurrent: nivolumab 360 mg d1, q3wks × 3; IMRT: 70 Gy, 33 fractions, 5 fractions/wk, 1 fraction/d | ||||||
| Adjuvant: nivolumab 480 mg d1; q4wks × 6 | ||||||
| Ma et al. (NCT03427827) | III | 417 | Yes | Stage III–IVA (except T3–4N0 and T3N1) | Induction: gemcitabine 1,000 mg/m2 d1, d8; cisplatin 80 mg/m2 d1; q3wks × 3 | Induction: gemcitabine 1,000 mg/m2 d1, d8; cisplatin 80 mg/m2 d1; q3wks × 3; Concurrent: cisplatin 100 mg/m2 d1; q3wks × 2; q3wks × 3; IMRT: 70 Gy, 6–7 wks |
| Concurrent: cisplatin 100 mg/m2 d1; q3wks × 2; q3wks × 3; IMRT: 70 Gy, 6–7 wks | ||||||
| Adjuvant: camrelizumab 3 mg/kg (≤200 mg) d1, q4wks × 12 | ||||||
| Ma et al. (NCT03700476) | III | 417 | Yes | Stage III–IVA (except T3–4N0 and T3N1) | Induction: gemcitabine 1,000 mg/m2 d1, d8; cisplatin 80 mg/m2 d1; sintilimab 200 mg d1, q3wks × 3 | Induction: gemcitabine 1,000 mg/m2 d1, d8; cisplatin 80 mg/m2 d1; q3wks × 3; Concurrent: cisplatin 100 mg/m2 d1; q3wks × 2; q3wks × 3; IMRT: 70 Gy, 6–7 wks |
| Concurrent: cisplatin 100 mg/m2 d1, sintilimab 200 mg d1, q3wks × 3; IMRT: 70 Gy, 6–7 wks | ||||||
| Adjuvant: sintilimab 200 mg d1, q3wks × 6 | ||||||
| Mell et al. (NCT03383094) | II | 114 | Yes | stage III–IVB (T1–2N2–3M0 or T3–4N0–3M0) p16+ squamous cell nasopharyngeal carcinoma | Concurrent: pembrolizumab 200 mg q3wks × 3; IMRT: 70 Gy, 33-35 fractions, 6.5 wks | Concurrent: cisplatin 100 mg q3wks × 3; IMRT: 70 Gy, 33–35 fractions, 6.5 wks |
| Adjuvant: pembrolizumab 200 mg q3wks × 17 | ||||||
| Yom et al. (NCT03267498) | II | 40 | Yes | stage II–IV; WHO type II/III | Induction: nivolumab 240 mg, d1 | NA |
| Concurrent: nivolumab 240 mg, d1, q2wks ×11; cisplatin 40 mg/m2, d1, q1wk × 22; RT: 7 0Gy, 33 fractions, 5 d/wk | ||||||
| Lu et al. (NCT04143984) | II/III | 180 | No | Recurrent non-metastatic; completed a definitive course of IMRT to a total dose of ≥66 Gy | Arm-C: Concurrent: camrelizumab 200 mg, q2wks × 27; carbon-ion radiotherapy: 63–69 GyE, 21–23 fractions | Carbon-ion radiotherapy: 63–69 GyE, 21–23 fractions |
| Arm-CA: Concurrent: camrelizumab 200 mg, q2wks × 27; apatinib, 250 mg, qd × 365; carbon-ion radiotherapy: 63–69 GyE, 21–23 fractions | ||||||
| NCT03734809 | II | 46 | Yes | Stage IVA; WHO type II/III | Induction: pembrolizumab 200 mg, q3wks × 2; gemcitabine-cisplatin | NA |
| Concurrent: pembrolizumab 200 mg, q3wks × 3; cisplatin; IMRT | ||||||
| Adjuvant: pembrolizumab 200 mg, q3wks × 12 |
NA, not applicable; PD-L1, programmed death-ligand 1; q2/3/4wks, every 2/3/4 weeks; d1, day 1; wks, weeks; RT, radiotherapy; IMRT, intensity-modulated radiotherapy.
Acknowledgments
Funding: This work was supported by grants from the National Natural Science Foundation of China (81930072), the Key-Area Research and Development Program of Guangdong Province (2019B020230002), the Natural Science Foundation of Guangdong Province (2017A030312003), the Health & Medical Collaborative Innovation Project of Guangzhou City, China (201803040003), the Innovation Team Development Plan of the Ministry of Education (No. IRT_17R110), and the Overseas Expertise Introduction Project for Discipline Innovation (111 Project, B14035).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Wai Tong Ng, Shao Hui Huang, Hai-Qiang Mai) for the series “Precision Radiotherapy in Nasopharyngeal Carcinoma” published in Annals of Nasopharynx Cancer. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/anpc-20-10). The series “Precision Radiotherapy in Nasopharyngeal Carcinoma” was commissioned by the editorial office without any funding or sponsorship. Jun Ma serves as an unpaid editorial board member of Annals of Nasopharynx Cancer from Aug 2019 to Aug 2021. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Yu WM, Hussain SS. Incidence of nasopharyngeal carcinoma in Chinese immigrants, compared with Chinese in China and South East Asia J Laryngol Otol 2009;123:1067-74. review. [Crossref] [PubMed]
- Wei KR, Zheng RS, Zhang SW, et al. Nasopharyngeal carcinoma incidence and mortality in China, 2013. Chin J Cancer 2017;36:90. [Crossref] [PubMed]
- Wei KR, Zheng RS, Zhang SW, et al. Nasopharyngeal carcinoma incidence and mortality in China in 2010. Chin J Cancer 2014;33:381-7. [PubMed]
- Xia C, Yu X, Zheng R, et al. Spatial and temporal patterns of nasopharyngeal carcinoma mortality in China, 1973-2005. Cancer Lett 2017;401:33-8. [Crossref] [PubMed]
- Xu ZJ, Zheng RS, Zhang SW, et al. Nasopharyngeal carcinoma incidence and mortality in China in 2009. Chin J Cancer 2013;32:453-60. [Crossref] [PubMed]
- World Health Organization. WHO Mortality Database. Available online: https://www.who.int/healthinfo/statistics/mortality_rawdata.
- Chen YP, Chan A, Le QT, et al. Nasopharyngeal carcinoma. Lancet 2019;394:64-80. [Crossref] [PubMed]
- DE Mekie, Lawley M. Nasopharyngeal carcinoma. I. Clinical analysis of one hundred twenty cases. AMA Arch Surg 1954;69:841-8. [Crossref] [PubMed]
- Moss WT. Therapeutic radiology: rationale, technique, results: Mosby 1965.
- Lee AW, Poon YF, Foo W, et al. Retrospective analysis of 5037 patients with nasopharyngeal carcinoma treated during 1976-1985: Overall survival and patterns of failure. Int J Radiat Oncol Biol Phys 1992;23:261-70. [Crossref] [PubMed]
- Geara FB, Sanguineti G, Tucker SL, et al. Carcinoma of the nasopharynx treated by radiotherapy alone: determinants of distant metastasis and survival. Radiother Oncol 1997;43:53-61. [Crossref] [PubMed]
- Lee AW, Sze WM, Au JS, et al. Treatment results for nasopharyngeal carcinoma in the modern era: the Hong Kong experience. Int J Radiat Oncol Biol Phys 2005;61:1107-16. [Crossref] [PubMed]
- Kam MK, Teo PM, Chau RM, et al. Treatment of nasopharyngeal carcinoma with intensity-modulated radiotherapy: the Hong Kong experience. Int J Radiat Oncol Biol Phys 2004;60:1440-50. [Crossref] [PubMed]
- Lee N, Xia P, Quivey J, et al. Intensity-modulated radiotherapy in the treatment of nasopharyngeal carcinoma: an update of the UCSF experience. Int J Radiat Oncol Biol Phys 2002;53:12-22. [Crossref] [PubMed]
- Wolden SL, Chen WC, Pfister DG, et al. Intensity-modulated radiation therapy (IMRT) for nasopharynx cancer: update of the Memorial Sloan-Kettering experience. Int J Radiat Oncol Biol Phys 2006;64:57-62. [Crossref] [PubMed]
- Bucci M, Xia P, Lee N, et al. Intensity modulated radiation therapy for carcinoma of the nasopharynx: An update of the UCSF experience. Int J Radiat Oncol Biol Phys 2004;60:S317-8. [Crossref]
- Lee SW, Back G, Yi B, et al. Preliminary results of a phase I/II study of simultaneous modulated accelerated radiotherapy for nondisseminated nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 2006;65:152-60. [Crossref] [PubMed]
- Wu S, Xie C, Jin X, et al. Simultaneous modulated accelerated radiation therapy in the treatment of nasopharyngeal cancer: A local center’s experience. Int J Radiat Oncol Biol Phys 2006;66:S40-6. [Crossref]
- Koom WS, Kim TH, Shin KH, et al. SMART (simultaneous modulated accelerated radiotherapy) for locally advanced nasopharyngeal carcinomas. Head Neck 2008;30:159-69. [Crossref] [PubMed]
- Moon SH, Cho KH, Lee CG, et al. IMRT vs. 2D-Strahlentherapie oder konformaler 3D-Strahlentherapie beim Nasopharynxkarzinom Folgen für das Überleben in einer koreanischen multizentrischen retrospektiven Studie (KROG 11-06). Strahlenther Onkol 2016;192:377-85. [Crossref] [PubMed]
- Bailet JW, Mark RJ, Abemayor E, et al. Nasopharyngeal carcinoma: treatment results with primary radiation therapy. Laryngoscope 1992;102:965-72. [Crossref] [PubMed]
- Chu AM, Flynn MB, Achino E, et al. Irradiation of nasopharyngeal carcinoma: Correlations with treatment factors and stage. Int J Radiat Oncol Biol Phys 1984;10:2241-9. [Crossref] [PubMed]
- Hoppe RT, Goffinet D, Bagshaw M. Carcinoma of the nasopharynx. Eighteen years’ experience with megavoltage radiation therapy. Cancer 1976;37:2605-12. [Crossref] [PubMed]
- Lee AW, Lau WH, Tung SY, et al. Preliminary results of a randomized study on therapeutic gain by concurrent chemotherapy for regionally-advanced nasopharyngeal carcinoma: NPC-9901 Trial by the Hong Kong Nasopharyngeal Cancer Study Group. J Clin Oncol 2005;23:6966-75. [Crossref] [PubMed]
- Mesic JB, Fletcher GH, Goepfert H. Megavoltage irradiation of epithelial tumors of the nasopharynx. Int J Radiat Oncol Biol Phys 1981;7:447-53. [Crossref] [PubMed]
- Ng WT, Lee MC, Hung WM, et al. Clinical outcomes and patterns of failure after intensity-modulated radiotherapy for nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 2011;79:420-8. [Crossref] [PubMed]
- Xiao WW, Huang SM, Han F, et al. Local control, survival, and late toxicities of locally advanced nasopharyngeal carcinoma treated by simultaneous modulated accelerated radiotherapy combined with cisplatin concurrent chemotherapy: long-term results of a phase 2 study. Cancer 2011;117:1874-83. [Crossref] [PubMed]
- Zhang B, Mo Z, Du W, et al. Intensity-modulated radiation therapy versus 2D-RT or 3D-CRT for the treatment of nasopharyngeal carcinoma: A systematic review and meta-analysis. Oral Oncol 2015;51:1041-6. [Crossref] [PubMed]
- Peng G, Wang T, Yang KY, et al. A prospective, randomized study comparing outcomes and toxicities of intensity-modulated radiotherapy vs. conventional two-dimensional radiotherapy for the treatment of nasopharyngeal carcinoma. Radiother Oncol 2012;104:286-93. [Crossref] [PubMed]
- Kam MK, Leung SF, Zee B, et al. Prospective randomized study of intensity-modulated radiotherapy on salivary gland function in early-stage nasopharyngeal carcinoma patients. J Clin Oncol 2007;25:4873-9. [Crossref] [PubMed]
- Liu W, Tsai K, Chien J, et al. The Impact of Image Guided Radiation Therapy for Nasopharyngeal Cancer Patients. Int J Radiat Oncol Biol Phys 2018;102:e332 [Crossref]
- Duma MN, Kampfer S, Wilkens JJ, et al. Comparative analysis of an image-guided versus a non-image-guided setup approach in terms of delivered dose to the parotid glands in head-and-neck cancer IMRT. Int J Radiat Oncol Biol Phys 2010;77:1266-73. [Crossref] [PubMed]
- Chen AM, Daly ME, Cui J, et al. Clinical outcomes among patients with head and neck cancer treated by intensity-modulated radiotherapy with and without adaptive replanning. Head Neck 2014;36:1541-6. [Crossref] [PubMed]
- Lewis GD, Holliday EB, Kocak-Uzel E, et al. Intensity-modulated proton therapy for nasopharyngeal carcinoma: Decreased radiation dose to normal structures and encouraging clinical outcomes. Head Neck 2016;38:E1886-95. [Crossref] [PubMed]
- Taheri-Kadkhoda Z, Björk-Eriksson T, Nill S, et al. Intensity-modulated radiotherapy of nasopharyngeal carcinoma: a comparative treatment planning study of photons and protons. Radiat Oncol 2008;3:4. [Crossref] [PubMed]
- Widesott L, Pierelli A, Fiorino C, et al. Intensity-modulated proton therapy versus helical tomotherapy in nasopharynx cancer: planning comparison and NTCP evaluation. Int J Radiat Oncol Biol Phys 2008;72:589-96. [Crossref] [PubMed]
- Cozzi L, Fogliata A, Lomax A, et al. A treatment planning comparison of 3D conformal therapy, intensity modulated photon therapy and proton therapy for treatment of advanced head and neck tumours. Radiother Oncol 2001;61:287-97. [Crossref] [PubMed]
- Alterio D, D’Ippolito E, Vischioni B, et al. Mixed-beam approach in locally advanced nasopharyngeal carcinoma: IMRT followed by proton therapy boost versus IMRT-only. Evaluation of toxicity and efficacy. Acta Oncol 2020;59:541-8. [Crossref] [PubMed]
- Beddok A, Feuvret L, Noel G, et al. Efficacy and toxicity of proton with photon radiation for locally advanced nasopharyngeal carcinoma. Acta Oncol 2019;58:472-4. [Crossref] [PubMed]
- Holliday E, Garden A, Rosenthal D, et al. Proton Therapy Reduces Treatment-Related Toxicities for Patients with Nasopharyngeal Cancer: A Case-Match Control Study of Intensity-Modulated Proton Therapy and Intensity-Modulated Photon Therapy. Int J Part Ther 2015;2:19-28. [Crossref]
- Lin R, Slater J, Yonemoto L, et al. Nasopharyngeal carcinoma: repeat treatment with conformal proton therapy—dose-volume histogram analysis. Radiology 1999;213:489-94. [Crossref] [PubMed]
- Chan A, Adams J, Weyman E, et al. A Phase II Trial of Proton Radiation Therapy With Chemotherapy for Nasopharyngeal Carcinoma. Int J Radiat Oncol Biol Phys 2012;84:S151-2. [Crossref]
- Wang L, Hu J, Liu X, et al. Intensity-modulated carbon-ion radiation therapy versus intensity-modulated photon-based radiation therapy in locally recurrent nasopharyngeal carcinoma: a dosimetric comparison. Cancer Manag Res 2019;11:7767-77. [Crossref] [PubMed]
- Akbaba S, Held T, Lang K, et al. Bimodal Radiotherapy with Active Raster-Scanning Carbon Ion Radiotherapy and Intensity-Modulated Radiotherapy in High-Risk Nasopharyngeal Carcinoma Results in Excellent Local Control. Cancers (Basel) 2019;11:379. [Crossref] [PubMed]
- Roeder F, Zwicker F, Saleh-Ebrahimi L, et al. Intensity modulated or fractionated stereotactic reirradiation in patients with recurrent nasopharyngeal cancer. Radiat Oncol 2011;6:22. [Crossref] [PubMed]
- Kong L, Hu J, Gao J, et al. Phase I/II Trial Evaluating Carbon-Ion Radiotherapy for Salvage Treatment of Locally Recurrent Nasopharyngeal Carcinoma. Int J Radiat Oncol Biol Phys 2019;105:E391-2. [Crossref]
- Hu J, Bao C, Gao J, et al. Salvage treatment using carbon ion radiation in patients with locoregionally recurrent nasopharyngeal carcinoma: Initial results. Cancer 2018;124:2427-37. [Crossref] [PubMed]
- Malouff TD, Mahajan A, Krishnan S, et al. Carbon Ion Therapy: A Modern Review of an Emerging Technology. Front Oncol 2020;10:82. [Crossref] [PubMed]
- Tao CJ, Yi JL, Chen NY, et al. Multi-subject atlas-based auto-segmentation reduces interobserver variation and improves dosimetric parameter consistency for organs at risk in nasopharyngeal carcinoma: A multi-institution clinical study. Radiother Oncol 2015;115:407-11. [Crossref] [PubMed]
- Lin L, Dou Q, Jin YM, et al. Deep Learning for Automated Contouring of Primary Tumor Volumes by MRI for Nasopharyngeal Carcinoma. Radiology 2019;291:677-86. [Crossref] [PubMed]
- National Comprehensive Cancer Network. Head and Neck Cancer. Available online: https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf.
- Li XY, Chen QY, Sun XS, et al. Ten-year outcomes of survival and toxicity for a phase III randomised trial of concurrent chemoradiotherapy versus radiotherapy alone in stage II nasopharyngeal carcinoma. Eur J Cancer 2019;110:24-31. [Crossref] [PubMed]
- Xu C, Zhang LH, Chen YP, et al. Chemoradiotherapy Versus Radiotherapy Alone in Stage II Nasopharyngeal Carcinoma: A Systemic Review and Meta-analysis of 2138 Patients. J Cancer 2017;8:287-97. [Crossref] [PubMed]
- Liu DH, Zhou XY, Pan YG, et al. Survival of stage II nasopharyngeal carcinoma patients with or without concurrent chemotherapy: A propensity score matching study. Cancer Med 2020;9:1287-97. [Crossref] [PubMed]
- Lin JC, Jan JS, Hsu CY, et al. Phase III study of concurrent chemoradiotherapy versus radiotherapy alone for advanced nasopharyngeal carcinoma: positive effect on overall and progression-free survival. J Clin Oncol 2003;21:631-7. [Crossref] [PubMed]
- Chan AT, Leung SF, Ngan RK, et al. Overall survival after concurrent cisplatin-radiotherapy compared with radiotherapy alone in locoregionally advanced nasopharyngeal carcinoma. J Natl Cancer Inst 2005;97:536-9. [Crossref] [PubMed]
- Kwong DL, Sham JS, Au GK, et al. Concurrent and adjuvant chemotherapy for nasopharyngeal carcinoma: a factorial study. J Clin Oncol 2004;22:2643-53. [Crossref] [PubMed]
- Wu X, Huang P, Peng P, et al. Long-term follow-up of a phase III study comparing radiotherapy with or without weekly oxaliplatin for locoregionally advanced nasopharyngeal carcinoma. Ann Oncol 2013;24:2131-6. [Crossref] [PubMed]
- Chen L, Hu CS, Chen XZ, et al. Concurrent chemoradiotherapy plus adjuvant chemotherapy versus concurrent chemoradiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma: a phase 3 multicentre randomised controlled trial. Lancet Oncol 2012;13:163-71. [Crossref] [PubMed]
- Chen L, Hu CS, Chen XZ, et al. Adjuvant chemotherapy in patients with locoregionally advanced nasopharyngeal carcinoma: Long-term results of a phase 3 multicentre randomised controlled trial. Eur J Cancer 2017;75:150-8. [Crossref] [PubMed]
- Tang LQ, Chen DP, Guo L, et al. Concurrent chemoradiotherapy with nedaplatin versus cisplatin in stage II-IVB nasopharyngeal carcinoma: an open-label, non-inferiority, randomised phase 3 trial. Lancet Oncol 2018;19:461-73. [Crossref] [PubMed]
- Al-Sarraf M, LeBlanc M, Giri P, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized Intergroup study 0099. J Clin Oncol 1998;16:1310-7. [Crossref] [PubMed]
- Chan A, Ngan R, Teo P, et al. Final results of a phase III randomized study of concurrent weekly cisplatin-RT versus RT alone in locoregionally advanced nasopharyngeal carcinoma (NPC). J Clin Oncol 2004;22:5523. [Crossref]
- Blanchard P, Lee A, Marguet S, et al. Chemotherapy and radiotherapy in nasopharyngeal carcinoma: an update of the MAC-NPC meta-analysis. Lancet Oncol 2015;16:645-55. [Crossref] [PubMed]
- He Y, Guo T, Guan H, et al. Concurrent chemoradiotherapy versus radiotherapy alone for locoregionally advanced nasopharyngeal carcinoma in the era of intensity-modulated radiotherapy: a meta-analysis. Cancer Manag Res 2018;10:1419-28. [Crossref] [PubMed]
- Songthong A, Chakkabat C, Kannarunimit D, et al. Efficacy of intensity-modulated radiotherapy with concurrent carboplatin in nasopharyngeal carcinoma. Radiol Oncol 2015;49:155-62. [Crossref] [PubMed]
- Chitapanarux I, Lorvidhaya V, Kamnerdsupaphon P, et al. Chemoradiation comparing cisplatin versus carboplatin in locally advanced nasopharyngeal cancer: randomised, non-inferiority, open trial. Eur J Cancer 2007;43:1399-406. [Crossref] [PubMed]
- Ke LR, Xia WX, Qiu WZ, et al. Safety and efficacy of lobaplatin combined with 5-fluorouracil as first-line induction chemotherapy followed by lobaplatin-radiotherapy in locally advanced nasopharyngeal carcinoma: preliminary results of a prospective phase II trial. BMC Cancer 2017;17:134. [Crossref] [PubMed]
- Wong AS, Soo RA, Lu JJ, et al. Paclitaxel, 5-fluorouracil and hydroxyurea concurrent with radiation in locally advanced nasopharyngeal carcinoma. Ann Oncol 2006;17:1152-7. [Crossref] [PubMed]
- Liao JF, Zhang Q, Du XJ, et al. Concurrent chemoradiotherapy with weekly docetaxel versus cisplatin in the treatment of locoregionally advanced nasopharyngeal carcinoma: a propensity score-matched analysis. Cancer Commun (Lond) 2019;39:40. [Crossref] [PubMed]
- Kwong DLW, Sham JST, Au GKH. Five-year Update on a Randomized Factorial Study on Concurrent and Adjuvant Chemotherapy for Advanced Nasopharyngeal Carcinoma. Int J Radiat Oncol Biol Phys 2006;66:S15-6. [Crossref]
- Yu Y, Liang H, Lv X, et al. Platinum-based concurrent chemotherapy remains the optimal regimen for nasopharyngeal carcinoma: a large institutional-based cohort study from an endemic area. J Cancer Res Clin Oncol 2018;144:2231-43. [Crossref] [PubMed]
- Peng L, Xu C, Chen YP, et al. Optimizing the cumulative cisplatin dose during radiotherapy in nasopharyngeal carcinoma: Dose-effect analysis for a large cohort. Oral Oncol 2019;89:102-6. [Crossref] [PubMed]
- Chi KH, Chang YC, Guo WY, et al. A phase III study of adjuvant chemotherapy in advanced nasopharyngeal carcinoma patients. Int J Radiat Oncol Biol Phys 2002;52:1238-44. [Crossref] [PubMed]
- Wee J, Tan E, Tai B, et al. Randomized trial of radiotherapy versus concurrent chemoradiotherapy followed by adjuvant chemotherapy in patients with American Joint Committee on Cancer/International Union against cancer stage III and IV nasopharyngeal cancer of the endemic variety. J Clin Oncol 2005;23:6730-8. [Crossref] [PubMed]
- Lee AWM, Tung SY, Ng WT, et al. A multicenter, phase 3, randomized trial of concurrent chemoradiotherapy plus adjuvant chemotherapy versus radiotherapy alone in patients with regionally advanced nasopharyngeal carcinoma: 10-year outcomes for efficacy and toxicity. Cancer 2017;123:4147-57. [Crossref] [PubMed]
- Lee AWM, Tung SY, Ng WT, et al. Randomized trial of radiotherapy plus concurrent-adjuvant chemotherapy vs radiotherapy alone for regionally advanced nasopharyngeal carcinoma. J Natl Cancer Inst 2010;102:1188-98. [Crossref] [PubMed]
- Lee AWM, Tung SY, Chan A, et al. A randomized trial on addition of concurrent-adjuvant chemotherapy and/or accelerated fractionation for locally-advanced nasopharyngeal carcinoma. Radiother Oncol 2011;98:15-22. [Crossref] [PubMed]
- Chen Y, Sun Y, Liang SB, et al. Progress report of a randomized trial comparing long-term survival and late toxicity of concurrent chemoradiotherapy with adjuvant chemotherapy versus radiotherapy alone in patients with stage III to IVB nasopharyngeal carcinoma from endemic regions of China. Cancer 2013;119:2230-8. [Crossref] [PubMed]
- Chua DT, Sham JS, Choy D, et al. Preliminary report of the Asian-Oceanian Clinical Oncology Association randomized trial comparing cisplatin and epirubicin followed by radiotherapy versus radiotherapy alone in the treatment of patients with locoregionally advanced nasopharyngeal carcinoma. Asian-Oceanian Clinical Oncology Association Nasopharynx Cancer Study Group. Cancer 1998;83:2270-83. [Crossref] [PubMed]
- Ma J, Mai H, Hong M, et al. Results of a prospective randomized trial comparing neoadjuvant chemotherapy plus radiotherapy with radiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma. J Clin Oncol 2001;19:1350-7. [Crossref] [PubMed]
- Lee AW, Tung SY, Ngan RK, et al. Factors contributing to the efficacy of concurrent-adjuvant chemotherapy for locoregionally advanced nasopharyngeal carcinoma: combined analyses of NPC-9901 and NPC-9902 Trials. Eur J Cancer 2011;47:656-66. [Crossref] [PubMed]
- Tan T, Lim WT, Fong KW, et al. Concurrent chemo-radiation with or without induction gemcitabine, Carboplatin, and Paclitaxel: a randomized, phase 2/3 trial in locally advanced nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 2015;91:952-60. [Crossref] [PubMed]
- Sun Y, Li WF, Chen NY, et al. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase 3, multicentre, randomised controlled trial. Lancet Oncol 2016;17:1509-20. [Crossref] [PubMed]
- Yang Q, Cao SM, Guo L, et al. Induction chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: long-term results of a phase III multicentre randomised controlled trial. Eur J Cancer 2019;119:87-96. [Crossref] [PubMed]
- Cao SM, Yang Q, Guo L, et al. Neoadjuvant chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: A phase III multicentre randomised controlled trial. Eur J Cancer 2017;75:14-23. [Crossref] [PubMed]
- Li WF, Chen NY, Zhang N, et al. Concurrent chemoradiotherapy with/without induction chemotherapy in locoregionally advanced nasopharyngeal carcinoma: Long-term results of phase 3 randomized controlled trial. Int J Cancer 2019;145:295-305. [Crossref] [PubMed]
- Hong RL, Hsiao CF, Ting LL, et al. Final results of a randomized phase III trial of induction chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in patients with stage IVA and IVB nasopharyngeal carcinoma-Taiwan Cooperative Oncology Group (TCOG) 1303 Study. Ann Oncol 2018;29:1972-9. [Crossref] [PubMed]
- Frikha M, Auperin A, Tao Y, et al. A randomized trial of induction docetaxel-cisplatin-5FU followed by concomitant cisplatin-RT versus concomitant cisplatin-RT in nasopharyngeal carcinoma (GORTEC 2006-02). Ann Oncol 2018;29:731-6. [Crossref] [PubMed]
- Zhang Y, Chen L, Hu G, et al. Gemcitabine and Cisplatin Induction Chemotherapy in Nasopharyngeal Carcinoma. N Engl J Med 2019;381:1124-35. [Crossref] [PubMed]
- Xu T, Zhu G, He X, et al. A phase III randomized study comparing neoadjuvant chemotherapy with concurrent chemotherapy combined with radiotherapy for locoregionally advanced nasopharyngeal carcinoma: updated long-term survival outcomes. Oral Oncol 2014;50:71-6. [Crossref] [PubMed]
- Xu T, Hu C, Zhu G, et al. Preliminary results of a phase III randomized study comparing chemotherapy neoadjuvantly or concurrently with radiotherapy for locoregionally advanced nasopharyngeal carcinoma. Med Oncol 2012;29:272-8. [Crossref] [PubMed]
- Liang ZG, Chen XQ, Lin GX, et al. Significant survival benefit of adjuvant chemotherapy after concurrent chemoradiotherapy in locally advanced high-risk nasopharyngeal carcinoma. Sci Rep 2017;7:41449. [Crossref] [PubMed]
- Hui EP, Ma BB, Chan KC, et al. Clinical utility of plasma Epstein-Barr virus DNA and ERCC1 single nucleotide polymorphism in nasopharyngeal carcinoma. Cancer 2015;121:2720-9. [Crossref] [PubMed]
- Lee VH, Kwong DL, Leung TW, et al. Prognostication of serial post-intensity-modulated radiation therapy undetectable plasma EBV DNA for nasopharyngeal carcinoma. Oncotarget 2017;8:5292-308. [Crossref] [PubMed]
- Twu CW, Wang WY, Chen CC, et al. Metronomic adjuvant chemotherapy improves treatment outcome in nasopharyngeal carcinoma patients with postradiation persistently detectable plasma Epstein-Barr virus deoxyribonucleic acid. Int J Radiat Oncol Biol Phys 2014;89:21-9. [Crossref] [PubMed]
- Chan ATC, Hui EP, Ngan RKC, et al. Analysis of Plasma Epstein-Barr Virus DNA in Nasopharyngeal Cancer After Chemoradiation to Identify High-Risk Patients for Adjuvant Chemotherapy: A Randomized Controlled Trial. J Clin Oncol 2018; [Crossref] [PubMed]
- Hui E, Li W, Ma BBY, et al. Development and validation of a risk model integrating plasma Epstein-Barr virus DNA (EBV DNA) level and TNM stage for stratification of nasopharyngeal cancer (NPC) to adjuvant therapy. Ann Oncol 2019;30:ix97-8. [Crossref]
- Kareva I, Waxman D, Lakka Klement G. Metronomic chemotherapy: an attractive alternative to maximum tolerated dose therapy that can activate anti-tumor immunity and minimize therapeutic resistance. Cancer Lett 2015;358:100-6. [Crossref] [PubMed]
- Chen JH, Huang WY, Ho CL, et al. Evaluation of oral tegafur-uracil as metronomic therapy following concurrent chemoradiotherapy in patients with non-distant metastatic TNM stage IV nasopharyngeal carcinoma. Head Neck 2019;41:3775-82. [Crossref] [PubMed]
- Feng M, Gao Y, Lang J, et al. A Phase II Prospective Study about the Efficacy and Toxicity of the Locally Advanced Nasopharyngeal Carcinoma Patients Treated with Concurrent Chemoradiotherapy Followed with the Capecitabine Metronomic Chemotherapy. Int J Radiat Oncol Biol Phys 2018;102:e262 [Crossref]
- Chua DT, Ma J, Sham JS, et al. Long-term survival after cisplatin-based induction chemotherapy and radiotherapy for nasopharyngeal carcinoma: a pooled data analysis of two phase III trials. J Clin Oncol 2005;23:1118-24. [Crossref] [PubMed]
- Ribassin-Majed L, Marguet S, Lee AWM, et al. What Is the Best Treatment of Locally Advanced Nasopharyngeal Carcinoma? An Individual Patient Data Network Meta-Analysis. J Clin Oncol 2017;35:498-505. [Crossref] [PubMed]
- Xue F, Hu C, He X. Induction chemotherapy followed by intensity-modulated radiotherapy with reduced gross tumor volume delineation for stage T3-4 nasopharyngeal carcinoma. Onco Targets Ther 2017;10:3329-36. [Crossref] [PubMed]
- International Nasopharynx Cancer Study Group. Preliminary results of a randomized trial comparing neoadjuvant chemotherapy (cisplatin, epirubicin, bleomycin) plus radiotherapy vs. radiotherapy alone in stage IV (or = N2, M0) undifferentiated nasopharyngeal carcinoma: a positive effect on progression-free survival. Int J Radiat Oncol Biol Phys 1996;35:463-9. [Crossref] [PubMed]
- Geara FB, Glisson BS, Sanguineti G, et al. Induction chemotherapy followed by radiotherapy versus radiotherapy alone in patients with advanced nasopharyngeal carcinoma: results of a matched cohort study. Cancer 1997;79:1279-86. [Crossref] [PubMed]
- Hareyama M, Sakata K, Shirato H, et al. A prospective, randomized trial comparing neoadjuvant chemotherapy with radiotherapy alone in patients with advanced nasopharyngeal carcinoma. Cancer 2002;94:2217-23. [Crossref] [PubMed]
- He X, Xu K, Guo J, et al. A meta-analysis of neoadjuvant chemotherapy plus radiation in the treatment of locally advanced nasopharyngeal carcinoma. J Cancer Res Ther 2015;11:C205-8. [Crossref] [PubMed]
- Li WF, Li YQ, Chen L, et al. Propensity-matched analysis of three different chemotherapy sequences in patients with locoregionally advanced nasopharyngeal carcinoma treated using intensity-modulated radiotherapy. BMC Cancer 2015;15:810. [Crossref] [PubMed]
- Xu C, Sun R, Tang LL, et al. Role of sequential chemoradiotherapy in stage II and low-risk stage III-IV nasopharyngeal carcinoma in the era of intensity-modulated radiotherapy: A propensity score-matched analysis. Oral Oncol 2018;78:37-45. [Crossref] [PubMed]
- Gabani P, Barnes J, Lin A, et al. Induction chemotherapy in the treatment of nasopharyngeal carcinoma: Clinical outcomes and patterns of care. Cancer Med 2018;7:3592-603. [Crossref] [PubMed]
- Huang PY, Cao KJ, Guo X, et al. A randomized trial of induction chemotherapy plus concurrent chemoradiotherapy versus induction chemotherapy plus radiotherapy for locoregionally advanced nasopharyngeal carcinoma. Oral Oncol 2012;48:1038-44. [Crossref] [PubMed]
- Komatsu M, Tsukuda M, Matsuda H, et al. Comparison of concurrent chemoradiotherapy versus induction chemotherapy followed by radiation in patients with nasopharyngeal carcinoma. Anticancer Res 2012;32:681-6. [PubMed]
- Wu SY, Wu YH, Yang MW, et al. Comparison of concurrent chemoradiotherapy versus neoadjuvant chemotherapy followed by radiation in patients with advanced nasopharyngeal carcinoma in endemic area: experience of 128 consecutive cases with 5 year follow-up. BMC Cancer 2014;14:787. [Crossref] [PubMed]
- Hui EP, Ma BB, Leung SF, et al. Randomized phase II trial of concurrent cisplatin-radiotherapy with or without neoadjuvant docetaxel and cisplatin in advanced nasopharyngeal carcinoma. J Clin Oncol 2009;27:242-9. [Crossref] [PubMed]
- Fountzilas G, Ciuleanu E, Bobos M, et al. Induction chemotherapy followed by concomitant radiotherapy and weekly cisplatin versus the same concomitant chemoradiotherapy in patients with nasopharyngeal carcinoma: a randomized phase II study conducted by the Hellenic Cooperative Oncology Group (HeCOG) with biomarker evaluation. Ann Oncol 2012;23:427-35. [Crossref] [PubMed]
- Chen YP, Tang LL, Yang Q, et al. Induction Chemotherapy plus Concurrent Chemoradiotherapy in Endemic Nasopharyngeal Carcinoma: Individual Patient Data Pooled Analysis of Four Randomized Trials. Clin Cancer Res 2018;24:1824-33. [Crossref] [PubMed]
- Tan T, Soon Y, Cheo T, et al. Addition of Induction Chemotherapy in Locally Advanced Nasopharyngeal Carcinoma Treated with Concurrent Chemo-Radiotherapy: A Meta-Analysis and Meta-Regression of Randomized Trials. Int J Radiat Oncol Biol Phys 2019;105:E395 [Crossref]
- Colevas AD, Yom SS, Pfister DG, et al. NCCN Guidelines Insights: Head and Neck Cancers, Version 1.2018. J Natl Compr Canc Netw 2018;16:479-90. [Crossref] [PubMed]
- Kong L, Zhang Y, Hu C, et al. Effects of induction docetaxel, platinum, and fluorouracil chemotherapy in patients with stage III or IVA/B nasopharyngeal cancer treated with concurrent chemoradiation therapy: Final results of 2 parallel phase 2 clinical trials. Cancer 2017;123:2258-67. [Crossref] [PubMed]
- Posner MR, Hershock DM, Blajman CR, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med 2007;357:1705-15. [Crossref] [PubMed]
- Zhou R, Zhu J, Chen X, et al. The efficacy and safety of docetaxel, cisplatin and fluorouracil (TPF)-based induction chemotherapy followed by concurrent chemoradiotherapy for locoregionally advanced nasopharyngeal carcinoma: a meta-analysis. Clin Transl Oncol 2020;22:429-39. [Crossref] [PubMed]
- Zhang L, Huang Y, Hong S, et al. Gemcitabine plus cisplatin versus fluorouracil plus cisplatin in recurrent or metastatic nasopharyngeal carcinoma: a multicentre, randomised, open-label, phase 3 trial. Lancet 2016;388:1883-92. [Crossref] [PubMed]
- Guan H, He Y, Wei Z, et al. Assessment of induction chemotherapy regimen TPF vs GP followed by concurrent chemoradiotherapy in locally advanced nasopharyngeal carcinoma: A retrospective cohort study of 160 patients. Clin Otolaryngol 2020;45:274-9. [Crossref] [PubMed]
- Fang W, Zhang J, Hong S, et al. EBV-driven LMP1 and IFN-γ up-regulate PD-L1 in nasopharyngeal carcinoma: Implications for oncotargeted therapy. Oncotarget 2014;5:12189-202. [Crossref] [PubMed]
- Larbcharoensub N, Mahaprom K, Jiarpinitnun C, et al. Characterization of PD-L1 and PD-1 Expression and CD8+ Tumor-infiltrating Lymphocyte in Epstein-Barr Virus-associated Nasopharyngeal Carcinoma. Am J Clin Oncol 2018;41:1204-10. [Crossref] [PubMed]
- Zhu Q, Cai MY, Chen CL, et al. Tumor cells PD-L1 expression as a favorable prognosis factor in nasopharyngeal carcinoma patients with pre-existing intratumor-infiltrating lymphocytes. Oncoimmunology 2017;6:e1312240 [Crossref] [PubMed]
- Zhang J, Fang W, Qin T, et al. Co-expression of PD-1 and PD-L1 predicts poor outcome in nasopharyngeal carcinoma. Med Oncol 2015;32:86. [Crossref] [PubMed]
- Hui EP, Taylor GS, Jia H, et al. Phase I trial of recombinant modified vaccinia ankara encoding Epstein-Barr viral tumor antigens in nasopharyngeal carcinoma patients. Cancer Res 2013;73:1676-88. [Crossref] [PubMed]
- Chia WK, Teo M, Wang WW, et al. Adoptive T-cell transfer and chemotherapy in the first-line treatment of metastatic and/or locally recurrent nasopharyngeal carcinoma. Mol Ther 2014;22:132-9. [Crossref] [PubMed]
- Chia WK, Wang WW, Teo M, et al. A phase II study evaluating the safety and efficacy of an adenovirus-ΔLMP1-LMP2 transduced dendritic cell vaccine in patients with advanced metastatic nasopharyngeal carcinoma. Ann Oncol 2012;23:997-1005. [Crossref] [PubMed]
- Louis CU, Straathof K, Bollard CM, et al. Adoptive transfer of EBV-specific T cells results in sustained clinical responses in patients with locoregional nasopharyngeal carcinoma. J Immunother 2010;33:983-90. [Crossref] [PubMed]
- Deng L, Liang H, Burnette B, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest 2014;124:687-95. [Crossref] [PubMed]
- Zeng J, See A, Phallen J, et al. Anti-PD-1 Blockade and Stereotactic Radiation Produce Long-Term Survival in Mice With Intracranial Gliomas. Int J Radiat Oncol Biol Phys 2013;86:343-9. [Crossref] [PubMed]
- Liu S, Sun X, Luo J, et al. Effects of radiation on T regulatory cells in normal states and cancer: mechanisms and clinical implications. Am J Cancer Res 2015;5:3276-85. [PubMed]
- Nagasaka M, Zaki M, Kim H, et al. PD1/PD-L1 inhibition as a potential radiosensitizer in head and neck squamous cell carcinoma: a case report. J Immunother Cancer 2016;4:83. [Crossref] [PubMed]
- Hsu C, Lee SH, Ejadi S, et al. Safety and Antitumor Activity of Pembrolizumab in Patients With Programmed Death-Ligand 1-Positive Nasopharyngeal Carcinoma: Results of the KEYNOTE-028 Study. J Clin Oncol 2017;35:4050-6. [Crossref] [PubMed]
- Ma BBY, Lim WT, Goh BC, et al. Antitumor Activity of Nivolumab in Recurrent and Metastatic Nasopharyngeal Carcinoma: An International, Multicenter Study of the Mayo Clinic Phase 2 Consortium (NCI-9742). J Clin Oncol 2018;36:1412-8. [Crossref] [PubMed]
- Fang W, Yang Y, Ma Y, et al. Camrelizumab (SHR-1210) alone or in combination with gemcitabine plus cisplatin for nasopharyngeal carcinoma: results from two single-arm, phase 1 trials. Lancet Oncol 2018;19:1338-50. [Crossref] [PubMed]
Cite this article as: Zhu GL, Xu C, Ma J. Combination of precision radiotherapy with chemotherapy and immunotherapy in non-recurrent/metastatic nasopharyngeal carcinoma. Ann Nasopharynx Cancer 2020;4:5.


