
Salivary glands and dental complications after radiotherapy for nasopharyngeal carcinoma
All nasopharyngeal cancer (NPC) required radiotherapy (RT) to both sides of the neck to cover the bilateral cervical lymph nodes. This resulted in a larger volume of salivary glands being irradiated and accounted for late complications to themselves and also to the nearby organs, e.g., teeth, mandible. RT may cause permanent damage to these structures and put the patients at a lifelong risk of complications. Decreasing of salivary function and flow can affect the quality of life of patients, i.e., problems in chewing and swallowing, difficulties in speech, mouth discomfort, taste alteration, oral infection, and dental caries. This review aims to summarize the development of late complications to the salivary glands and teeth following RT for NPC. Additionally, we also provide prevention strategies and the management of these late side effects.
Salivary glands complication after RT for nasopharyngeal carcinoma
Damage to the salivary glands is a major concern with RT for NPC. All of the major saliva glands (parotid/submandibular/sublingual) are the crucial glands for producing saliva. They produce 70–80% of the total saliva. Minor salivary glands scattered all over the oral cavity and pharynx produce a residual of 20–30% (1-3). Parotid glands produce a watery texture and high protein concentration saliva. Parotid glands are also the biggest salivary gland and very sensitive to radiation. Damage to this gland plays a key role in the generation of late suffering sequelae to NPC patients.
Lubrication and protection from the microorganisms of the oral cavity are the key roles of saliva. Other functions included preservation of the equilibrium of dental minerals, keeping the acid-base balance, and maintain hygiene in the oral cavity. Digestive activity is among the major functions of saliva. High dose RT delivered to the salivary glands can damage the structure of salivary gland tissue. The glands are substituted and infiltrated by the lymphocytes, plasma cells, and fibrous connective tissue. As a result, glands become atrophic and fibrotic then the production of saliva by glands ceases (4,5).
Radiation sensitivity of salivary glands is varied due to the mixture of serous acinar and mucous cells in their structure, also the division property in each gland. The serous acinar cells of the parotid glands are subsequently the most vulnerable to RT after the serous acinar cells of submandibular glands (6). Several days after the commencement of RT, xerostomia, or dry mouth feeling is the first and most frequent symptom of patients. There was a report of decreasing saliva production as high to 50% within 24 hours after RT initiation even with a low dose of 225 cGy (6). If the entire salivary glands are irradiated with only 1,000 cGy, the saliva production can decrease over 50% and may cause sticky and gluey saliva for the whole week. With a higher dose to 6,000 cGy, the production rate declines greater than 75% (7,8). Contrary to several studies, they demonstrated that the volume of saliva production drops dramatically after 4,000 cGy of RT (9,10).
Dreizen (7) reported the decrease of saliva production from 83.3% to 44% and also lower saliva pH from 7.01 to 6.83 after 6 weeks of RT. Simultaneously the alteration pH of saliva, the increasing concentration of minerals in the saliva (sodium, chlorine, calcium, magnesium, and protein), and the decreasing concentration of bicarbonate were found in their study. The latter is the greatest key factor in creating acid-base equilibrium in saliva. Bacteria in the oral cavity and dental plaque produce acids that raid tooth enamel triggering dental cavities. Bicarbonate balances this acidity by increasing the pH of saliva.
Protecting the salivary glands during RT
Some investigators explored methods to prevent oral cavity dryness other than treatment interventions. One of the methods was the application of pilocarpine concurrent with RT. One concept proposed that the serous cell damage during RT causes leakage of granules within the cells consisting of proteolytic enzymes. They hypothesized that pilocarpine applied along with RT can avoid this damage, consequently lowering the amount of inter-cellular granules (11). The results obtained from this study did not support this theory (12). There are randomized controlled studies comparing patients who did and did not receive pilocarpine while being irradiated and demonstrated decreased mouth dryness symptoms together with increased salivary flow (13,14). Radiation Therapy Oncology Group study (RTOG-9709) (15) and Princess Margaret Hospital (PMH) (16) investigated the patients who received RT more than 50 Gy, covered more than 50% of the major saliva glands and randomly prescribed the placebo and 5 mg of pilocarpine three times daily during RT and continued up to 3 months after RT in the RTOG study while the patients studied by the PMH were given only 1 month after RT. In RTOG-9709, the saliva flow rate in patients using pilocarpine was higher with statistical significance on the last day of RT and 3 months later. However, no difference was shown at 6 months following the completion of RT. PMH study evaluated the efficacy of pilocarpine using questionnaires, and the results did not show any differences between the two groups of patients. Some further studies of the effects of this drug on the patients’ quality of life but no relations between saliva flow rates and the mouth dryness were seen.
A radioprotective agent which is called amifostine (WR-2721) has been reported as a potent agent to protect salivary glands during RT. It is organic thiophosphate which can be dephosphorylated by alkaline phosphate enzyme in the plasma membrane to an active metabolite (WR-1065). Subsequently, the effective substance WR-1065 will act as a free radical scavenger caused by ionizing radiation. Since alkali phosphate enzyme is hardly found in cancer cells in comparison to normal cells, the enzyme selects to protect the normal cells much more than the cancer cells. This could increase the therapeutic index of RT (17,18). This drug has a very short half-life, therefore it needs to be injected into the vein immediately before each session of RT, essentially not more than 15 minutes. The common side effects are nausea, vomiting, flushing, and low blood pressure. Brizel et al. (18) performed a large randomized study that compared amifostine 200 mg/m2 before each RT session with patients who were not prescribed any drugs. The subjective assessment of mouth dryness was evaluated through a questionnaire and the objective assessment was evaluated by measuring the salivary flow rate. Patients with Grade 2 or more xerostomia were significantly lower in the amifostine group. Besides, there was no difference in oral mucositis and the local control rate between the two groups. Anne et al. (19) compared another application form of this medication via subcutaneous injection instead of intravenous and found that both of them provide similar efficacy. The different side effects were that the subcutaneous route did not cause low blood pressure and had less severe vomiting.
Another strategy to protect salivary glands during RT is sparing some volume of them from getting a high dose of radiation by using sophisticated RT techniques. There are numerous studies with statistical significance about three dimensions of conformal RT (3D-CRT) as well as intensity-modulated RT (IMRT) to help partially preserving and decreasing the RT dose to the salivary glands (20-25). A systematic review by Gupta et al. (26) concluded that IMRT can significantly reduce the risk of serious acute and late xerostomia compared to conventional RT or 3D-CRT for patients receiving a curative dose of RT to the head and neck region. Eisbruch et al. (27) provided the subjective assessment of xerostomia through the questionnaires to IMRT bilateral neck irradiated patients versus patients irradiated using normal techniques. One year after RT, the score of mouth dryness in patients with IMRT technique was 3.1±0.19 compared with 5.1±0.2. The higher score was the worst symptom of mouth dryness. They concluded that the IMRT technique had more useful in decreasing the mouth dryness. Furthermore, they have demonstrated that 2 years after IMRT, the flow rate of saliva from protected parotid glands has been back to the same level as before being irradiated.
Proton, a newer sophisticated RT technique, has been studied in the prevention of late xerostomia (28). This study retrospectively compared the self-reported xerostomia-specific questionnaire between 425 oropharyngeal cancer (OPC) patients treated by IMRT and 104 OPC patients treated by intensity-modulated proton therapy (IMPT). They reported significant reduction of late xerostomia in the patients treated by IMPT due to decreasing the volume of the contralateral parotid gland.
Managing late radiation-induced xerostomia
Simulation of the salivary flow by chewing should be advised to the irradiated head and neck cancer patients with the symptom of mouth dryness. Dodds et al. demonstrated the increased saliva from parotid glands after 1 to 2 weeks of increasing mastication through daily gum chewing (29). Moreover, the pH of saliva increased to the appropriate level within the oral cavity (29). Using cholinergic parasympathomimetic agents such as pilocarpine is suggested to alleviate the symptom of dry mouth in the patients who still has a response to saliva stimulation. The study by Curry et al. reported the benefit of pilocarpine in enhancing the parotid function in 1964 (30) Since then many randomized studies supported that it is one of the successful management of xerostomia (30,31). The appropriate dose of pilocarpine was studied by LeVeque et al. (31). The efficacy and side effects of pilocarpine were tested with a starting dose at 2.5 mg and titrated to 10 mg, three times a day for 4 months. Another study by Johnson et al. (32) performed the randomized trial between pilocarpine versus placebo. Both studies used questionnaires to assess the efficacy of this drug (mouth dryness, speaking difficulty, oral cavity discomfort, chewing, swallowing, and denture wearing). All of these studies revealed that pilocarpine significantly improves xerostomia symptoms. Pilocarpine of 5 mg three times per day was the best dosage. It has not been demonstrated that using higher than 5 mg per dose produces better outcomes. The common side effects are sweating, nausea, palpitations, dizziness, and runny nose. Nevertheless, no study reported the permanent and long-lasting salivary flow. One study by Horiot et al. (33) reported that two-thirds of the patients in the pilocarpine group had significant xerostomia-related symptom improvement, especially in the swallowing. Based on these studies, pilocarpine is effective for at least 30–60% of these patients. Though, at least 3 to 4 weeks may be needed to see the efficacy of this drug. The dose can be doubled to 10 mg per dose if the dose of 5 mg is not effective. The period of 6–8 weeks follow-up should be made after prescribing this medicine. It must be avoided in patients who had clinically significant uncontrolled cardiac disease, uncontrolled asthma, closed-angle glaucoma, and other chronic diseases at risk for cholinergic agonists. The side effects must be monitored, especially in patients with hypertension, arrhythmia, renal disease, chronic obstructive pulmonary disease (COPD), asthma, or patients with a history of hypersensitivity to cholinergic agents.
We conducted a prospective study to test the efficacy of placebo versus pilocarpine in the same patient at different periods (34). Irradiated head and neck cancer patients who had parotid glands in the RT fields and received the dose of 5,000 cGy or more together with the symptoms of mouth dryness were included in this trial. All eligible patients received the placebo three times per day for the first month followed by pilocarpine 5 mg thrice a day for the second to fourth months. Subjective assessment was evaluated by using the questionnaires together with the objective assessment by two radiation oncologists at the end of each month. The symptoms of xerostomia were significantly improved in all 33 patients for the first time of using pilocarpine. This efficacy had still been found until the last follow-up time of the trial. Both subjective and objective measurements demonstrated that pilocarpine were significantly statistically better than placebo. The most common complaint was sweating. Other common side effects include nausea, palpitation, and lacrimation. However, the latest Cochrane Database Systematic Review (35) compared pilocarpine with no treatment or placebo in 12 trials. No differences were observed in xerostomia symptoms and the flow rate of saliva both stimulated and unstimulated between treatment groups at any time points (end of RT, 3 and 6 months). They have concluded that there was scarce evidence-based to support the beneficial impact of pilocarpine on improving quality of life or increasing survival. Cochrane Database Systematic Review (35) also reviewed the studies of palifermin in the management of xerostomia and concluded that there is insufficient evidence to determine whether or not palifermin reduced the incidence of Grade 2 or more xerostomia up to 3 months after RT. Data is still lacking to support the efficacy of this drug on overall or progression-free survival. In addition to pilocarpine and palifermin, there were several clinical studies of other interventions, e.g., artificial saliva, antiseptic mouth-wash, antimicrobial lozenge, bethanechol, and polaprezinc. Nonetheless, there was insufficient evidence to support their relevance (35). The latest systematic review concluded that acupuncture needs more evidence for support as an evidence-based treatment option for dry mouth symptoms from any causes (36).
Patients who developed severe late xerostomia and no response to every saliva stimulation intervention will not get any benefit from pilocarpine as well. Moistening the oral cavity is the goal to alleviate the symptom of dry mouth. The cheapest and easiest intervention is frequently sipping water. Saliva substitutes or artificial saliva can be also used to moisturize the mouth. There are various types of artificial saliva; polymer-based, mucin-based, and carboxymethylcellulose (CMC)-based. These artificial saliva formulas can help lessen xerostomia symptoms. Though, the outcome of alleviating dry mouth between each formula did not seem to make a lot of difference. Visch et al. (37) compared the efficacy of a CMC- and mucin-containing artificial saliva. They concluded that patients preferred mucin-containing formulas to CMC-containing formulas and about 30% of the patients did not gain advantages from administration these saliva substitutes. Hatton et al. (38) have found that mucin-based saliva substitutes appear to lubricate better than those containing CMC and also similar to the human saliva. Other investigators also did not reveal any xerostomia symptom improvement when using these substitutes comparing to the placebo (39-41).
Complications of dental and related structures after RT
Head and neck RT can produce many complications to the teeth and related structures. As aforementioned, irradiated and damaged salivary glands lead to functional impairment of the glands, resulting in hyposalivation and xerostomia. Without enough saliva as protective fluid in the oral cavity, diseases of the teeth, periodontium, and oral mucosa occur more easily than those who have sufficient quantity and quality of saliva. In addition, ionizing radiation may directly affect the teeth, periodontal tissue, and taste buds, causing impairment of those tissues.
Dental caries
It has long been recognized that one of the earliest problems after RT is dental caries (42-44). Patients with head and neck cancer who have undergone irradiation may develop rampant carious dental lesions, known as radiation caries. Radiation caries has a rapid onset usually without any pain symptom. It can occur as soon as the first 3 months after RT. Radiation caries particularly affect the cervical aspects, incisal edges, and occlusal surfaces of teeth. As a result, the amputation of the tooth crowns often takes place. In severe cases, dental caries may progress into the pulp and periapical tissue, causing pulpitis and periapical lesions. Subsequently, the teeth may need extracting, leading to a future risk of osteoradionecrosis (ORN). The etiologies of dental caries in irradiated patients include hyposalivation and a direct effect of the ionizing radiation. A recent in vitro study revealed that direct radiation on teeth could induce alterations of the mechanical properties, ultrastructures, and chemical composition of the hard tissue (45). Moreover, damage to the dentin enamel junction (DEJ) and reduced enamel crystallinity under irradiation was found to be related to the formation of radiation caries as described above. As the dose of radiation escalates, the tooth is more prone to direct destruction. The radiation doses that increase the risk of dental caries two to three times are between 30–60 Gy and the risk drastically increases ten times when the dose is higher than 60 Gy (46). A more recent cross-sectional study on the association between IMRT and tooth destruction on 42 patients with nasopharyngeal carcinoma who received IMRT was conducted (47). The results showed that new tooth damage occurred at doses of more than 35.8 Gy. It was suggested that radiation doses to less than 50 Gy should be planned for patients with nasopharyngeal carcinoma. Xerostomia is believed to play an even more significant role in dental caries in irradiated patients. Having a deficiency of the saliva, irradiated patients are impaired in tooth-cleansing effects and the process of tooth remineralization, leading to the accumulation of dental plaque and dental caries (40). Figure 1 demonstrated the patient with radiation caries.
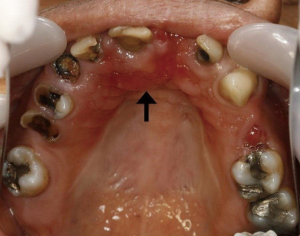
To prevent radiation caries, it is strongly recommended that all patients should receive comprehensive dental care and oral health instruction before the course of RT (42,43). All dental cavities even the early white spot lesions should be completely restored or treated. To prevent dental caries, the most effective strategy is the application of fluoride to patients’ teeth in individual trays. Additional means include the use of fluoride varnishes, fluoride mouth rinses, high fluoride concentrated toothpaste, and fluoride slow-release devices.
After RT, all patients should be recalled every 6 months for a thorough oral examination to detect whether or not the patients are developing dental caries, periodontitis, or any oral mucosal lesions (42). Appropriate treatments should then be carried out in individual patients with apparent diseases. Short-term follow-up is recommended in patients with persistent hyposalivation.
ORN
ORN of the jaws is a severe, late-effect complication particularly of the mandible in irradiated patients with head and neck cancers (48,49). ORN of the jaws is defined as exposed bone caused by irradiation that fails to heal for more than 3 months without any persisting or recurrent neoplasms (50). ORN may occur spontaneously or be caused by trauma, particularly after tooth extraction. The incidence of ORN of the mandible falls in between 2–22% (51). Recent studies have reported that the prevalence of ORN significantly decreases due to many factors including the use of megavoltage RT, improved dental care, and improved radiation delivery for example IMRT (49,52). The onset of ORN often occurs from 4 months to 2 years. The risk, however, remains for life but with a diminished degree. Clinically, patients with ORN experience a painful symptom with exposed necrotic bone seen through ulcerated oral mucosa or skin. Other clinical findings include unpleasant sensation, halitosis, altered taste sensation, food impaction on the affected area, fistula opening from the oral mucosa or skin, infection, and pathologic fractures (51). Risk factors of ORN include total radiation dose more than 60 Gy, treatment with brachytherapy, concomitant chemo-radiation, fractionation, poor oral hygiene, poor nutritional status, alcohol, and tobacco uses, dental extractions, tumor size and location, and advanced stage tumor. To prevent ORN, extraction of the teeth that cannot be preserved for a long time should be considered 2–3 weeks before the initiation of RT (53). Teeth with periodontal pockets equal or more than 5 mm and teeth with furcation involvement should be extracted. Figure 2 demonstrates the patient with ORN of the mandible.
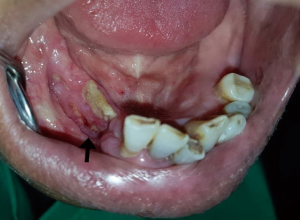
Management of ORN is always difficult and complicated due to the chronic and progressive nature of the disease. In mild cases, a conservative management including local irrigation with saline solution, NaHCO3 or 0.2% chlorhexidine, prescription of systemic antibiotics, avoidance of irritations, and oral hygiene instruction is recommended (52,53). Also, gentle removal of sequestrum or necrotic bone should be performed. In advanced stages of the disease for example in patients with intractable pain, pathologic fracture, or failure to conservative management, jaw resection and reconstruction with free flap should be initiated. In general, determination of the extent of jaw resection is based on the presence of bleeding at the surgical edges. Recurrences of ORN can occur even in cases where adequate resection is performed. Long-term follow-up of patients with ORN is, therefore, mandatory.
It has long been known that hyperbaric oxygen therapy (HBOT) may be used as an adjunct treatment in patients with advanced stages of ORN due to HBOT can stimulate fibroblast proliferation and collagen formation, and increase angiogenesis in bone tissue (53). However, previous studies have shown variable or even discordant results of the benefit of HBOT in ORN. An earlier systematic review showed the resolution rates of ORN with HBOT varied from 19–93% (54). A more recent Cochrane review concluded that HBOT appears to decrease the chance of ORN following tooth extraction (55). Currently, some on-going multicentre randomized controlled trials have been carried out to evaluate the benefit of HBOT with or without medications, such as tocopherol, pentoxifylline, and clodronate, in patients with ORN (53,56). Findings from these studies will certainly shed light on the beneficial role of HBOT in ORN.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Nasopharynx Cancer for the series “Late Complications in the Management of Nasopharyngeal Cancer”. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/anpc-20-17). The series “Late Complications in the Management of Nasopharyngeal Cancer” was commissioned by the editorial office without any funding or sponsorship. IC served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Annals of Nasopharynx Cancer from August 2019 to August 2021. The other author has no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Smith PM. Mechanisms of secretion by salivary glands. In: Edgar WM, O’Mullane DM. editors. Saliva and Oral Health. 2nd edition. London: BDJ, 1996:9-25.
- Shannon IL, Trodahl JN, Starcke EN. Radiosensitivity of the human parotid gland. Proc Soc Exp Biol Med 1978;157:50-3. [Crossref] [PubMed]
- Mira JG, Wescott WB, Starcke EN, et al. Some factors influencing salivary function when treating with radiotherapy. Int J Radiat Oncol Biol Phys 1981;7:535-41. [Crossref] [PubMed]
- Mossman KL. Quantitative radiation dose-response relationships for normal tissues in man. II. Response of the salivary glands during radiotherapy. Radiat Res 1983;95:392-8. [Crossref] [PubMed]
- Mossman K, Shatzman A, Chencharick J. Long-term effects of radiotherapy on taste and salivary function in man. Int J Radiat Oncol Biol Phys 1982;8:991-7. [Crossref] [PubMed]
- Sreebny L. Xerostomia: diagnosis, management and clinical complications. In: Edgar WM, O’Mullane DM. editors. Saliva and oral health, 2nd edition. London: BDJ, 1996:43-66.
- Dreizen S. Oral complications of cancer therapies. Description and incidence of oral complications. NCI Monogr 1990;11-5. [PubMed]
- Beumer J 3rd, Curtis T, Harrison RE. Radiation therapy of the oral cavity: sequelae and management, part 1. Head Neck Surg 1979;1:301-12. [Crossref] [PubMed]
- Vissink A, Panders AK, Johannes-Gravenmmade E, et al. The causes and consequences of hyposalivation. Ear Nose Throat J 1988;67:166-8, 173-6. [PubMed]
- Eneroth CM, Henrikson CO, Jakobsson PA. Effect of fractionated radiotherapy on salivary gland function. Cancer 1972;30:1147-53. [Crossref] [PubMed]
- Kim KH, Kim JY, Sung MW, et al. The effect of pilocarpin and atropin administration on radiation-induced injury of rat submandibular gland. Acta Otolaryngol 1991;111:967-73. [Crossref] [PubMed]
- Coppes RP, Zeilstra LJW, Vissink A, et al. Sialogoguerelated radioprotection of salivary gland function: The degranulation concept revisited. Radiat Res 1997;148:240-7. [Crossref] [PubMed]
- Valdez IH, Wolff A, Atkinson JC, et al. Use of pilocarpine during head and neck radiation therapy to reduce xerostomia and salivary dysfunction. Cancer 1993;71:1848-51. [Crossref] [PubMed]
- Zimmerman RP, Mark RJ, Tran LM, et al. Concomitant pilocarpine during head and neck irradiation is associated with decreased posttreatment xerostomia. Int J Radiat Oncol Biol Phys 1997;37:571-5. [Crossref] [PubMed]
- Scarantino C, LeVeque F, Swann RS, et al. Effect of pilocarpine during radiation therapy: results of RTOG 97-09, a phase III randomized study in head and neck cancer patients. J Support Oncol 2006;4:252-8. [PubMed]
- Hassan SJ, Weymuller EA. Assessment of quality of life in head and neck cancer patients. Head Neck 1993;15:485-96. [Crossref] [PubMed]
- Sodicoff M, Conger AD, Pratt NE, et al. Radioprotection by WR-2721 against long-term chronic damage in the rat parotid gland. Radiat Res 1978;76:172-9. [Crossref] [PubMed]
- Brizel DM, Wasserman TH, Henke M, et al. Phase III randomized trial of amifostine as a radioprotector in head and neck cancer. J Clin Oncol 2000;18:3339-45. Erratum in: J Clin Oncol 2000 Dec 15;18(24):4110-1. [Crossref] [PubMed]
- Anné PR, Curran WJ Jr. A phase II trial of subcutaneous amifostine and radiation therapy in patients with head and neck cancer. Semin Radiat Oncol 2002;12:18-9. [Crossref] [PubMed]
- Maes A, Weltens C, Flamen P, et al. Preservation of parotid function with uncomplicated conformal radiotherapy. Radiother Oncol 2002;63:203-11. [Crossref] [PubMed]
- Eisbruch A, Marsh LH, Martel MK, et al. Comprehensive irradiation of head and neck cancer using conformal multi-segmental fields: Assessment of target coverage and non-involved tissue sparing. Int J Radiat Oncol Biol Phys 1998;41:559-68. [Crossref] [PubMed]
- Wu Q, Manning M, Schmidt-Ullrich R, et al. The potential for sparing of parotids and escalation of biologically equivalent dose with intensity modulated radiation treatments of head and neck cancers: A treatment design study. Int J Radiat Oncol Biol Phys 2000;46:195-205. [Crossref] [PubMed]
- Chao KSC, Low D, Perez CA, et al. Intensity-modulated radiation therapy in head and neck cancer: The Mallincrodt experience. Int J Cancer 2000;90:92-103. [Crossref] [PubMed]
- Hunt MA, Zelefsky MJ, Wolden S, et al. Treatment planning and delivery of intensity-modulated radiation therapy for primary nasopharyngeal cancer. Int J Radiat Oncol Biol Phys 2001;49:623-32. [Crossref] [PubMed]
- Lee N, Xia P, Quivey JM, et al. Intensity modulated radiotherapy in the treatment of nasopharyngeal carcinoma: An update of the UCSF experience. Int J Radiat Oncol Biol Phys. 2002;53:12-22. [Crossref] [PubMed]
- Gupta T, Kannan S, Ghosh-Laskar S, et al. Systematic review and meta-analyses of intensity-modulated radiation therapy versus conventional two-dimensional and/or or three-dimensional radiotherapy in curative-intent management of head and neck squamous cell carcinoma. PLoS One 2018;13:e0200137 [Crossref] [PubMed]
- Eisbruch A, Kim HM, Terrell JE, et al. Xerostomia and its predictors following parotid-sparing irradiation of head and neck cancer. Int J Radiat Oncol Biol Phys 2001;50:695-704. [Crossref] [PubMed]
- Cao JZ, Zhang X, Jiang B, et al. The Significant Reduction of Late Xerostomia of IntensityModulated Proton Therapy for Oropharyngeal Cancer. Int J Radiat Biol Phys 2018;102:E207
- Dodds MWJ, Hseih SC, Johnson DA. The effect of increased mastication by daily gum chewing on salivary gland output and dental plaque acidogenicity. J Dent Res 1991;70:1474-8. [Crossref] [PubMed]
- Curry RC, Patey DH. A clinical test for parotid function. Br J Surg 1964;51:891-2. [Crossref] [PubMed]
- LeVeque FG, Montgomery M, Potter D. A multicenter, randomized, double-blind, placebo-controlled, dose-titration study of oral pilocarpine for treatment of radiation induced xerostomia in head and neck cancer patients. J Clin Oncol 1993;11:1124-31. [Crossref] [PubMed]
- Johnson JT, Ferretti GA, Nethery WJ. Oral pilocarpine for post-irradiation xerostomia in patients with head and neck cancer. N Engl J Med 1993;329:390-5. [Crossref] [PubMed]
- Horiot JC, Lipinski F, Schraub S, et al. Post-radiation severe xerostomia relieved by pilocarpine: A prospective French cooperative study. Radiother Oncol 2000;55:233-9. [Crossref] [PubMed]
- Chitapanarux I, Kamnerdsupaphon P, Tharavichitkul E, et al. Effect of oral pilocarpine on post-irradiation xerostomia in head and neck cancer patients: A single-center, single-blind clinical trial. J Med Assoc Thai 2008;91:1410-5. [PubMed]
- Riley P, Glenny AM, Hua F, et al. Pharmacological interventions for preventing dry mouth and salivary gland dysfunction following radiotherapy. Cochrane Database Syst Rev 2017;7:CD012744 [PubMed]
- Assy Z, Brand HS. A systematic review of the effects of acupuncture on xerostomia and hyposalivation. BMC Complement Altern Med 2018;18:57. [Crossref] [PubMed]
- Visch LL. A double-blind cross-over trial of CMC- and mucin-containing salivary substitutes. Int J Oral Maxillofac Surg 1986;15:395-400. [Crossref] [PubMed]
- Hatton MN, Levine MJ, Margarone JE, et al. Lubrication and viscosity features of human saliva and commercially available saliva substitutes. J Oral Maxillofac Surg 1987;45:496-9. [Crossref] [PubMed]
- Eisbruch A, Rhodus N, Rosenthal D, et al. How should we measure and report xerostomia? Semin Radiat Oncol 2003;13:226-34. [Crossref] [PubMed]
- Olsson H, Axell T. Objective and subjective efficacy of saliva substitutes containing mucin and carboxymethylcellulose. Scand J Dent Res 1991;99:316-9. [PubMed]
- Jellema AP, Langendijk H, Bergenhenegouwen L. The efficacy of Xialine in patients with xerostomia resulting from radiotherapy of head and neck cancer. Radiother Oncol 2001;59:157-60. [Crossref] [PubMed]
- Sroussi HY, Epstein JB, Bensadoun RJ, et al. Common oral complications of head and neck cancer radiation therapy: mucositis, infections, saliva change, fibrosis, sensory dysfunctions, dental caries, periodontal disease, and osteoradionecrosis. Cancer Med 2017;6:2918-31. [Crossref] [PubMed]
- Gupta N, Pal M, Rawat S, et al. Radiation-induced dental caries, prevention and treatment - A systematic review. Natl J Maxillofac Surg 2015;6:160-6. [Crossref] [PubMed]
- Dobroś K, Hajto-Bryk J, Wróblewska M, et al. Radiation-induced caries as the late effect of radiation therapy in the head and neck region. Contemp Oncol (Pozn) 2016;20:287-90. [Crossref] [PubMed]
- Lu H, Zhao Q, Guo J, et al. Direct radiation-induced effects on dental hard tissue. Radiat Oncol 2019;14:5. [Crossref] [PubMed]
- Walker MP, Wichman B, Cheng AL. Impact of Radiotherapy Dose on Dentition Breakdown in Head and Neck Cancer Patients. Pract Radiat Oncol 2011;1:142-8. [Crossref] [PubMed]
- Liang X, Zhang J, Peng G, et al. Radiation caries in nasopharyngeal carcinoma patients after intensity-modulated radiation therapy: A cross-sectional study. J Dent Sci 2016;11:1-7. [Crossref] [PubMed]
- Rathy R, Sunil S, Nivia M. Osteoradionecrosis of mandible: Case report with review of literature. Contemp Clin Dent 2013;4:251-3. [Crossref] [PubMed]
- Chronopoulos A, Zarra T, Ehrenfeld M, et al. Osteoradionecrosis of the jaws: definition, epidemiology, staging and clinical and radiological findings. A concise review. Int Dent J 2018;68:22-30. [Crossref] [PubMed]
- Teng MS, Futran ND. Osteoradionecrosis of the mandible. Curr Opin Otolaryngol Head Neck Surg 2005;13:217-21. [Crossref] [PubMed]
- Lyons A, Ghazali N. Osteoradionecrosis of the jaws: current understanding of its pathophysiology and treatment. Br J Oral Maxillofac Surg 2008;46:653-60. [Crossref] [PubMed]
- Nadella KR, Kodali RM, Guttikonda LK, et al. Osteoradionecrosis of the Jaws: Clinico-Therapeutic Management: A Literature Review and Update. J Maxillofac Oral Surg 2015;14:891-901. [Crossref] [PubMed]
- Raggio BS, Winters R. Modern management of osteoradionecrosis. Curr Opin Otolaryngol Head Neck Surg 2018;26:254-9. [Crossref] [PubMed]
- Peterson DE, Doerr W, Hovan A, et al. Osteoradionecrosis in cancer patients: the evidence base for treatment-dependent frequency, current management strategies, and future studies. Support Care Cancer 2010;18:1089-98. [Crossref] [PubMed]
- Bennett MH, Feldmeier J, Hampson NB, et al. Hyperbaric oxygen therapy for late radiation tissue injury. Cochrane Database Syst Rev 2016;4:CD005005 [PubMed]
- Dhanda J, Pasquier D, Newman L, et al. Current Concepts in Osteoradionecrosis after Head and Neck Radiotherapy. Clin Oncol (R Coll Radiol) 2016;28:459-66. [Crossref] [PubMed]
Cite this article as: Chitapanarux I, Iamaroon A. Salivary glands and dental complications after radiotherapy for nasopharyngeal carcinoma. Ann Nasopharynx Cancer 2020;4:7.


