Local radiotherapy combined with systemic therapy for metastatic nasopharyngeal carcinomas at diagnosis: experience from a tertiary academic center in the Philippines
Introduction
Nasopharyngeal carcinoma (NPC) is a relatively rare disease worldwide, but is endemic in certain parts of Southern China and Southeast Asia. It has been reported to be a rapidly proliferating disease, and is particularly well-known for its sensitivity to radiotherapy (RT) and chemotherapy (1,2). Current standard of care for locally advanced NPCs is concurrent chemoradiation (CRT), as established by the intergroup study of Al Sarraf et al. (3). Newer studies incorporating modern RT techniques with concurrent chemotherapy now report local control rates exceeding 90%, with most failures occurring at distant sites (4-7).
Metastatic NPC (mNPC) at diagnosis is reported to be even more uncommon, as it occurs only in about 6% of cases (8). mNPC is generally considered to be an incurable disease, and is associated with a poor prognosis. Several published series of mNPC have reported a median survival time of approximately 9 to 22 months (9-13). In these patients, systemic therapy is recommended as the first line treatment. However, the role of local RT, defined as RT to the primary site and regional nodal areas, still remains unclear in mNPC. Aggressive RT in this setting is controversial because of the unknown benefits when contrasted with the known toxicities associated with head and neck RT. Still, some studies have reported long-term survival of patients with mNPC, suggesting the possible value of a more aggressive treatment (14).
Recent studies have shown that in patients with mNPC, an overall survival (OS) benefit was found for those who had local RT in addition to systemic therapy compared to those who underwent chemotherapy alone (10,11,15,16). Interestingly, a case report of 5 patients with mNPC treated with local RT was able to show survival as long as 91 months (17). Toxicity data however, are still lacking in most studies and therefore, assessing the risk-benefit ratio of this treatment can be difficult.
Despite NPC being endemic in certain areas of Southeast Asia, no studies reporting outcomes after local RT for mNPC have been published from this region. Southeast Asia includes the Philippines, a low-to-middle income country (LMIC) that has been consistently reported to be endemic for NPC (18-21). Our institution is a tertiary academic center in the Philippines that treats approximately 30 to 40 new NPCs and one to two mNPCs annually at its Radiation Oncology department. Based on the study of Yoshida et al., our institution may be considered a high-volume facility for NPC (22). The objective of this study is to report survival outcomes and treatment-related toxicities after local RT for mNPC at the Benavides Cancer Institute – University of Santo Tomas Hospital. We present this article in accordance with the STROBE guideline checklist (available at http://dx.doi.org/10.21037/anpc-20-13).
Methods
Study design
This is a retrospective analysis of adult patients with mNPC treated at the Benavides Cancer Institute – University of Santo Tomas Hospital in Manila, Philippines, from 2006 to 2019. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of the University of Santo Tomas Hospital with protocol reference no. REC-2018-07-134-TR. Informed consent was not required by the review board since only chart review was done. From January 2006 to February 2019, all patients with biopsy-proven NPC presenting with distant metastasis at diagnosis (n=21) and treated with local RT at the Benavides Cancer Institute – University of Santo Tomas Hospital were included. Patients initially diagnosed with non-metastatic disease or those with mNPC treated with systemic treatment alone were excluded from the study. Patients’ charts were reviewed and data were tabulated in a tailored spreadsheet.
Treatment
All patients were evaluated with clinical history and physical examination and underwent flexible nasopharyngoscopy with biopsy. Imaging of the nasopharynx and neck (preferably MRI with contrast) and complete metastatic workup was done. All patients were staged using the American Joint Committee on Cancer (AJCC) 8th edition system. Patients staged using older systems were restaged using AJCC 8th edition.
Patients initially were simulated in the supine position and immobilized with a thermoplastic mask. Treatment volumes included all areas of gross disease (seen during physical and endoscopic examinations and imaging), the entire nasopharynx, anterior one-third to half of the clivus (or entire clivus if involved), skull base, pterygoid fossa, parapharyngeal space, sphenoid sinus, posterior ¼ of the nasal cavity and maxillary sinuses, inferior soft palate, retropharyngeal nodes, retrostyloid space, cavernous sinus (for T3 and T4) and bilateral neck nodes levels Ib through V. Treatment was delivered with either modified Ho’s (n=13) or intensity-modulated radiotherapy (IMRT) (n=8) technique. Total dose for most patients was 70 Gray (Gy) (1.8–2 Gy daily fractions) using modified Ho’s or 69.96 Gy (1.64–2.12 Gy daily fractions) using IMRT. Under the modified Ho’s technique, patients were initially treated with opposing lateral fields with matched anterior neck fields. The fields were sequentially shrunk by applying blocks to the spinal cord after 45 Gy, the sella after 54 Gy, the skull base after 60 Gy, with a final whole nasopharynx boost to 70 Gy after 66 Gy. A posterior electron field was added after initiating the spinal cord block. Simultaneous integrated boost (SIB) was used for the IMRT treated patients. A minority of patients were treated with palliative-intent RT (median 36 Gy, range 14 to 36 Gy) which was only given to the symptomatic tumor. Plans were performed using the Philips Pinnacle Treatment Planning System version 7.6c and delivered using a Siemens Primus linear accelerator.
Chemotherapy was administered as part of the treatment. Consensus on the sequence, the number of cycles, and schedule of chemotherapy were reached during the institute’s regular multidisciplinary head and neck tumor board meetings. Chemotherapy was given either as part of induction therapy, concurrent with RT or as palliative treatment. The following chemotherapy regimens were used: cisplatin with 5-fluorouracil (PF), docetaxel with cisplatin and 5-fluorouracil (TPF), gemcitabine with cisplatin (GP), and gemcitabine with carboplatin.
Assessment
The primary outcome measured was OS. The secondary outcome measured was treatment-related toxicities. For the assessment of outcomes, Patients were monitored weekly for toxicities and response during treatment. Once finished, patients were followed up at the clinic (including nasopharyngoscopy) every 3 months for the first 3 years, every 6 months during the 4th to 5th years, and annually thereafter. MRI or CT imaging of the nasopharynx and neck was done every 6 months to 1 year, or as clinically indicated. Acute toxicities were graded using the Radiation Therapy Oncology Group (RTOG) radiation morbidity grading system and the late toxicities were scored using the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 system.
Statistical analysis
Data from patient charts were encoded using Microsoft Excel and analysis was done using SPSS version 24. Missing data were handled using the last observer carried forward (LOCF) method. Survival curves was estimated using the Kaplan-Meier method. Univariate analysis of possible prognostic factors (age, performance status, T classification, N classification, number of metastasis, treatment modality used, total dose received, area of metastasis, RT technique, presence of liver metastasis) was done using the log rank method. A P-value of less than 0.05 was considered statistically significant.
Results
A total of 21 patients were included in this study. The patients and treatment characteristics are shown in Table 1. The median age of the cohort was 51. Sixty-two percent had an Eastern Cooperative Oncology Group (ECOG) performance status of ≤1. Almost all patients only had a single metastatic site, with bone metastases being the most common. Patients were treated using modified Ho’s (61.9%) and IMRT (38%) techniques, with the majority (81%) receiving a high dose (≥66 Gy) to the primary site. Palliative chemotherapy with RT, upfront CRT, or induction chemotherapy followed by CRT was given to 23.8%, 23.8%, and 52.4% of the patients, respectively. The most commonly used regimen for systemic therapy was PF while cisplatin alone was most commonly given in the concurrent regimen. There were no patients who received RT to metastatic sites at the time of diagnosis. Nine patients had missing follow up data which was handled using the LOCF method.
Table 1
| Characteristics | No. of patients (%) |
|---|---|
| Age in years | |
| >65 | 2 (9.5) |
| ≤65 | 19 (90.5) |
| Sex | |
| Male | 11 (52.4) |
| Female | 10 (47.6) |
| ECOG performance status | |
| ≤1 | 13 (61.9) |
| >1 | 8 (38.1) |
| T classification † | |
| T1 | 2 (9.5) |
| T2 | 7 (33.3) |
| T3 | 5 (23.8) |
| T4 | 7 (33.3) |
| N classification† | |
| N1 | 4 (19.0) |
| N2 | 3 (14.3) |
| N3 | 13 (61.9) |
| Number of metastatic sites | |
| Single | 19 (90.5) |
| Multiple | 2 (9.5) |
| Treatment received | |
| Induction chemotherapy + CRT | 11 (52.4) |
| Upfront CRT | 5 (23.8) |
| Palliative Chemotherapy + RT | 5 (23.8) |
| RT technique | |
| Modified Ho’s | 13 (61.9) |
| IMRT | 8 (38.1) |
| RT dose | |
| High dose (≥66 Gy) | 17 (84.2) |
| Low dose (<66 Gy) | |
| 36 Gy in 6 Fractions | 2 (9.5) |
| QUAD shot (14 Gy in 4 fractions) | 1 (4.8) |
| Did not continue after 42.4 Gy | 1 (4.8) |
| Systemic chemotherapy regimen | |
| Cisplatin/5-Fluorouracil (PF) | 14 (66.7) |
| Docetaxel/Cisplatin/5-fluorouracil (TPF) | 2 (9.5) |
| Area of metastasis | |
| Bone | 14 (66.7) |
| Liver | 4 (19.0) |
| Lungs | 2 (9.5) |
| Axillary nodes | 1 (4.8) |
| Skin | 1 (4.8) |
| Infraclavicular area | 1 (4.8) |
| Grade 3 or 4 toxicities | 5 (23.8) |
†, American Joint Committee on Cancer (AJCC) 8th edition. ECOG, Eastern Cooperative Oncology Group; T, tumor; N, node; CRT, chemoradiation; RT, radiotherapy; IMRT, intensity-modulated radiation therapy; Gy, Gray.
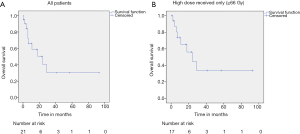
The median follow up time was 10 months (range, 1 to 93 months). The median survival for the entire cohort was 24 (95% CI, 4.98–43.01) months (Figure 1A). Median survival for those who received ≥66 Gy was 24 (95% CI, 8.30–39.70) months (Figure 1B). The one-, two-, and three-year OS rates were 61.8%, 42.4% and 31.8% respectively. Performance status, presence of liver metastases, number of metastatic sites, treatment received, and RT dose were found to be significant predictors of OS (Table 2).
Table 2
| Factor | Median Survival (months) | P-value |
|---|---|---|
| Age in years | ||
| ≤65 | 24; 95% CI, 5.21–42.79 | 0.218 |
| >65 | 4 | |
| ECOG performance status | ||
| ≤1 | 29; 95% CI, 14.77–43.23 | 0.005* |
| >1 | 6; 95% CI, 4.28–7.72 | |
| Treatment received | ||
| Induction Chemotherapy + CRT | 11; 95% CI, 0–30.50 | 0.032* |
| Upfront CRT | 29; 95% CI, 11.40–46.60 | |
| Palliative chemotherapy + RT | 3 | |
| RT technique | ||
| Modified Ho’s | 18; 95% CI, 2.07–33.93 | 0.161 |
| IMRT | 42 | |
| Number of metastatic sites | ||
| Single | 24; 95% CI, 5.18–42.82 | 0.002* |
| >1 metastatic sites | 1 | |
| Area of metastasis | ||
| Bone | 18; 95% CI, 0–38.30 | 0.441 |
| Visceral | 14 | |
| RT dose | ||
| High dose | 24; 95% CI, 8.30–40.00 | 0.04* |
| Low dose | 6 | |
| T classification† | ||
| T1–T2 | 18; 95% CI, 5.09–30.91 | 0.849 |
| T3–T4 | 24; 95% CI, 0–55.75 | |
| N classification† | ||
| N0–N1 | 29 | 0.235 |
| N2–N3 | 18; 95% CI, 1.55–34.45 | |
| Liver metastasis | ||
| Present | 6 | 0.023* |
| Absent | 24; 95% CI, 9.66–38.34 |
*, Statistically significant; †, American Joint Committee on Cancer (AJCC) 8th edition. ECOG, Eastern Cooperative Oncology Group; CRT, chemoradiation; RT, radiotherapy; IMRT, intensity-modulated radiation therapy; T, tumor; N, node.
Most patients tolerated the treatment well, with a median overall treatment time (OTT) of 52 days (range, 10 to 66 days) during the definitive RT phase. RTOG grade 3 mucositis was observed in 5 (26.3%) patients. No grade 4 toxicities were seen. Late toxicities, such as grade 2 xerostomia and grade 1 dysphagia, were observed in 3 patients (14.3%).
Discussion
In this retrospective study, we have found that patients with mNPC at diagnosis who were treated with local RT in addition to systemic chemotherapy had a median survival time of 24 (95% CI, 4.98–43.01) months. This is despite the inclusion of patients with poorer performance status (ECOG ≥2), unlike in prior published studies. Also, survival times as long as 93 months have been observed in our cohort, suggesting the possibility of long term survival in these patients. This outcome is similar to the survival data in other studies (Table 3). Rusthoven et al. reported median survival estimates of 21.4 months for those receiving local RT versus 15.5 months for those who did not (11). Another retrospective study by Lin et al. reported a median survival time of 25 months for patients who underwent local RT (23). A recent phase III randomized controlled trial from China (available only in abstract form) has demonstrated improved OS in patients receiving local RT along with chemotherapy versus patients treated with chemotherapy alone (24). Interestingly, similar survival benefit from local RT has also been demonstrated in other types of tumors like breast and prostate cancers (25-27).
Table 3
| Authors | Type of study | No. of patients | Median survival (months) | 1-year OS (%) | 2-year OS (%) |
|---|---|---|---|---|---|
| Current Cohort (2019) | Retrospective | 21 | 25 | 61.8 | 42.4 |
| Rusthoven et al. (2017) | Retrospective (Population based) | 437 | 21.4 | 67 | 50 |
| Verma et al. (2017) | Retrospective | 555 | 24.8 | 73 | 51 |
| Hu et al. (2017) | Retrospective (Population based) | 679 | 21 | 61.2 | 47.2 |
| Chen et al. (2013) | Retrospective | 590 | 24.7 | 79.2 | 51.7 |
| Lin et al. (2012) | Retrospective | 105 | 25 | 67.5 | 50.3 |
| Yeh et al. (2006) | Retrospective | 125 | 9.7 | 39 | 14 |
| Yin et al. (2017) | Retrospective | 611 | 20 | 95.3 | 75.2 |
mNPC, metastatic nasopharyngeal carcinoma.
The current National Comprehensive Cancer Network (NCCN) guidelines for the treatment of mNPC patients at diagnosis recommend the administration of combination chemotherapy first, and only adding local RT after a complete response to primary chemotherapy (28). Upfront concurrent CRT can be considered in those with low tumor burden, oligometastatic disease, or those with symptoms resulting from the primary site.
Although the sample size was limited, our univariate analysis showed the following factors to be predictors for improved survival after local RT: good performance status (ECOG ≤1), receipt of upfront CRT, single site metastasis only, treatment to high dose RT, and the absence of liver metastases. These factors that predicted for improved survival was also seen in other studies (11,12,23). Although not seen in our cohort, age < 65 years and lower nodal stage (N0-1) were also found to be significantly associated with better OS in prior published reports (11-13,17). These factors may aid the selection of patients with mNPC who are candidates for more aggressive treatment. It may therefore be hypothesized that local therapy may be more appropriate in patients with the above-mentioned favorable prognostic factors, whereas patients expected to have a poor prognosis may be spared the added toxicity of more aggressive treatment.
Most of the patients in this study received PF as their chemotherapeutic regimen. Recent studies have shown improved outcomes with the use of more aggressive combination therapy, which may be applicable in the subset of patients with better performance status. Jin et al. compared different cisplatin-based regimens for mNPC and reported better tumor response with GP and the TPF regimen compared to PF; however, OS and progression-free survival (PFS) were similar in both regimens (29). A more recent phase III trial comparing GP versus PF for mNPC reported improved PFS (HR 0.55; P<0.0001) and OS (HR 0.62; P=0.0025) with the use of GP chemotherapy versus PF (30). Because of these studies, the current preferred first-line chemotherapy regimen for mNPC is the GP combination.
The survival outcomes for patients with good performance status in our cohort compares rather favorably with that of the publications using PF chemotherapy (Table 4). In fact, our data more closely approximates Zhang’s study which utilizes a more currently accepted regimen (GP) (31). Whether the addition of local therapy to the GP combination and/or immunotherapy can further improve outcomes in mNPC remains an unanswered question. It can be argued that the improvement in distant control with modern chemotherapy may increase future emphasis on local control, with the increased utilization of RT to improve disease control. Besides offering the potential for improvement of symptoms from the primary site, the use of RT may also provide excellent local control as seen in previous non-metastatic NPC trials. Al-Sarraf et al. had local control rates of 67% in the RT alone and 90% in the CRT group with a median follow up of 2.7 years (3). Using a similar protocol, the study by Wee et al. had local relapse rates of 7.2% in the RT alone arm and 5.4% at 3 years (7). On the other hand, it is also possible that these new therapies may already provide sufficient local control in patients with metastatic disease even without RT.
Table 4
| Authors | Chemotherapy regimen | No. of patients | Median survival (months) | 1-year OS (%) | 2-year OS (%) |
|---|---|---|---|---|---|
| Current Cohort (2019). (ECOG ≤1) | Cisplatin/5FU | 21 | 29 | 79.5 | 53 |
| Zhang et al. (2019) | Gemcitabine/Cisplatin | 181 | 29.1 | 83.4 | 54.5 |
| Cisplatin/5FU | 181 | 20.9 | 72 | 42 | |
| Jin et al. (2012) | Cisplatin/5FU | 176 | 19.5 | 77 | 39 |
| Paclitaxel/Cisplatin | 167 | 21 | 74 | 41 | |
| Gemcitabine/Cisplatin | 173 | 21.5 | 82 | 46 | |
| Bleomycin/Cisplatin/5FU | 152 | 19 | 78 | 35 | |
| Paclitaxel/Cisplatin/5FU | 154 | 21 | 79 | 45 |
mNPC, metastatic nasopharyngeal carcinoma; 5FU, 5-Fluorouracil; ECOG, Eastern Cooperative Oncology Group.
The current emergence of cancer immunotherapy has significantly improved the management of advanced cancers. It has already been established as standard of care in certain malignancies (31,32) and is currently being tested in other types of cancer including NPC. A phase II trial by Ma et al. reported significant clinical benefit using nivolumab for heavily treated metastatic or recurrent NPCs, showing an objective response rate of 20.5% (33). The PACIFIC-NPC study is currently an ongoing trial for the use of camrelizumab in locally advanced NPCs (34). Results of these trials can possibly alter the landscape in the treatment of mNPC, both systemically and locally.
The majority of patients in this cohort received RT via the modified Ho’s technique. With the current use of IMRT, it is now possible to give higher doses to the tumor with better sparing of the organs at risk, producing a better therapeutic ratio (35). The use of more stringent image guidance may further improve the ratio. These developments may provide further benefit for mNPC patients, wherein the potential for improved survival must be carefully balanced with the considerable toxicities and adverse effects on quality of life that is associated with more aggressive treatment.
Patients with mNPC are generally considered to be incurable; hence, RT in this setting, even with higher doses, is traditionally given with palliative intent. To date, there is still no consensus regarding the optimal RT dose in this subset of patients. One retrospective study showed that RT doses >65 Gy are significantly associated with better OS (23). This was not observed in our cohort, which may be attributed to the small sample size. Grade 3 to 4 toxicity occurred in 26.3% of our patients, which resolved after conservative management. There was also no significant prolongation of OTT, suggesting that local RT is a relatively tolerable treatment modality for mNPC patients provided that they are closely monitored.
Whether or not more aggressive treatment to the metastatic foci in the oligometastatic setting is warranted is also currently a subject of discussion and debate. Metastatic disease has been documented to seed future metastasis in prostate cancer (36). Whether or not addressing distant disease by means of stereotactic ablative body radiotherapy (SABR) will further improve survival in these patients may be a subject of interest in future mNPC trials.
As of the authors’ knowledge, this is the only study to date that reports on mNPC local therapy outcomes from an LMIC in the Asia Pacific. Our findings at least suggest that results similar to that of more developed countries are achievable in LMICs, as long as patients are treated in a multidisciplinary setting. Although it is preferable to offer IMRT to mNPC patients being considered for local therapy, it may still not be widely available in less developed countries. In these scenarios, our study suggests that local RT given even via conventional techniques is feasible, with good tolerability.
This study is inherently limited by its retrospective nature, along with our relatively small sample size. Data regarding response and compliance to chemotherapy were not available. It was also unknown whether patients received further chemotherapy after CRT, which can be an important factor given the chemosensitivity of NPC.
Conclusions
Our results suggest that definitive local RT can potentially improve OS in patients with mNPC at diagnosis. Results of this study along with other published data suggest that it is reasonable to consider local RT in a carefully selected subset of patients. Specifically, local RT may be contemplated in patients with good performance status, those without liver metastases and/or those with a single metastatic site only.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/anpc-20-13
Data Sharing Statement: Available at http://dx.doi.org/10.21037/anpc-20-13
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/anpc-20-13). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional/regional/national ethics/committee/ethics board of University of Santo Tomas Hospital (PHREB accreditation No. L3-2016-020-01). Informed consent was not required by the review board since only chart review was done.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Skinner DW, Van Hasselt CA, Tsao SY. Nasopharyngeal carcinoma: modes of presentation. Ann Otol Rhinol Laryngol 1991;100:544-51. [Crossref] [PubMed]
- Chan ATC, Teo PML, Huang DP. Pathogenesis and treatment of nasopharyngeal carcinoma. Semin Oncol 2004;31:794-801. [Crossref] [PubMed]
- Al-Sarraf M, LeBlanc M, Giri PG, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized Intergroup study 0099. J Clin Oncol 1998;16:1310-7. [Crossref] [PubMed]
- Ng WT, Lee MCH, Hung WM, et al. Clinical outcomes and patterns of failure after intensity-modulated radiotherapy for nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 2011;79:420-8. [Crossref] [PubMed]
- Jiang F, Jin T, Feng XL, et al. Long-term outcomes and failure patterns of patients with nasopharyngeal carcinoma staged by magnetic resonance imaging in intensity-modulated radiotherapy era: The Zhejiang Cancer Hospital’s experience. J Cancer Res Ther 2015;11:C179-84. [Crossref] [PubMed]
- Lee N, Harris J, Garden AS, et al. Intensity-modulated radiation therapy with or without chemotherapy for nasopharyngeal carcinoma: radiation therapy oncology group phase II trial 0225. J Clin Oncol 2009;27:3684-90. [Crossref] [PubMed]
- Wee J, Tan EH, Tai BC, et al. Randomized trial of radiotherapy versus concurrent chemoradiotherapy followed by adjuvant chemotherapy in patients with American Joint Committee on Cancer/International Union against cancer stage III and IV nasopharyngeal cancer of the endemic variety. J Clin Oncol 2005;23:6730-8. [Crossref] [PubMed]
- Teo PM, Kwan WH, Lee WY, et al. Prognosticators determining survival subsequent to distant metastasis from nasopharyngeal carcinoma. Cancer 1996;77:2423-31. [Crossref] [PubMed]
- Loong HH, Ma BB, Chan AT. Update on the management and therapeutic monitoring of advanced nasopharyngeal cancer. Hematol Oncol Clin North Am 2008;22:1267-78. x. [Crossref] [PubMed]
- Hu J, Kong L, Gao J, et al. Use of Radiation Therapy in Metastatic Nasopharyngeal Cancer Improves Survival: A SEER Analysis. Sci Rep 2017;7:721. [Crossref] [PubMed]
- Rusthoven CG, Lanning RM, Jones BL, et al. Metastatic nasopharyngeal carcinoma: Patterns of care and survival for patients receiving chemotherapy with and without local radiotherapy. Radiother Oncol 2017;124:139-46. [Crossref] [PubMed]
- Yeh SA, Tang Y, Lui CC, et al. Treatment outcomes of patients with AJCC stage IVC nasopharyngeal carcinoma: benefits of primary radiotherapy. Jpn J Clin Oncol 2006;36:132-6. [Crossref] [PubMed]
- Chen MY, Jiang R, Guo L, et al. Locoregional radiotherapy in patients with distant metastases of nasopharyngeal carcinoma at diagnosis. Chin J Cancer 2013;32:604-13. [Crossref] [PubMed]
- Fandi A, Bachouchi M, Azli N, et al. Long-term disease-free survivors in metastatic undifferentiated carcinoma of nasopharyngeal type. J Clin Oncol 2000;18:1324-30. [Crossref] [PubMed]
- Verma V, Allen PK, Simone CB 2nd, et al. Addition of Definitive Radiotherapy to Chemotherapy in Patients With Newly Diagnosed Metastatic Nasopharyngeal Cancer. J Natl Compr Canc Netw 2017;15:1383-91. [Crossref] [PubMed]
- Yin Z, Zhang X, Wang Y, et al. The combination of systemic therapy and locoregional radiotherapy prolongs survival in newly diagnosed metastatic nasopharyngeal carcinoma patients. Onco Targets Ther 2017;10:5677-83. [Crossref] [PubMed]
- Setton J, Wolden S, Caria N, et al. Definitive treatment of metastatic nasopharyngeal carcinoma: Report of 5 cases with review of literature. Head Neck 2012;34:753-7. [Crossref] [PubMed]
- Chan J, Bray F, McCarron P, et al. Nasopharyngeal carcinoma. World Health Organization Classification of Tumours Pathology and Genetics of Head and Neck Tumor. Lyon, France: IARC Press, 2005:85-97.
- Laudico AV, Mirasol-Lumague MR, Mapua CA, et al. 2010 Philippine Cancer Facts and Estimates. Manila: Philippine Cancer Society, Inc., 2010:63-64.
- Sarmiento MPCB, Mejia MBA. Preliminary assessment of nasopharyngeal carcinoma incidence in the Philippines: a second look at published data from four centers. Chin J Cancer 2014;33:159-64. [Crossref] [PubMed]
- Agas RAF, Yu KKL, Sogono PG, et al. Reirradiation for Recurrent Nasopharyngeal Carcinomas: Experience From an Academic Tertiary Center in a Low- to Middle-Income Country. J Glob Oncol 2019;5:1-14. [Crossref] [PubMed]
- Yoshida EJ, Luu M, David JM, et al. Facility Volume and Survival in Nasopharyngeal Carcinoma. Int J Radiat Oncol Biol Phys 2018;100:408-17. [Crossref] [PubMed]
- Lin S, Tham IWK, Pan J, et al. Combined high-dose radiation therapy and systemic chemotherapy improves survival in patients with newly diagnosed metastatic nasopharyngeal cancer. Am J Clin Oncol 2012;35:474-9. [Crossref] [PubMed]
- Chen M, You R, You-Ping L. Chemotherapy plus local-regional radiotherapy versus chemotherapy alone in primary metastatic nasopharyngeal carcinoma: A randomized, open-label, phase III trial. Ann Oncol 2019;30:v449-4. [Crossref]
- Cho Y, Chang JS, Rha KH, et al. Does Radiotherapy for the Primary Tumor Benefit Prostate Cancer Patients with Distant Metastasis at Initial Diagnosis? PloS One 2016;11:e0147191 [Crossref] [PubMed]
- Le Scodan R, Stevens D, Brain E, et al. Breast cancer with synchronous metastases: survival impact of exclusive locoregional radiotherapy. J Clin Oncol 2009;27:1375-81. [Crossref] [PubMed]
- Parker CC, James ND, Brawley CD, et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet 2018;392:2353-66. [Crossref] [PubMed]
- National Comprehensive Cancer Network. (2019). Head and Neck Cancer (version 2.2019). Available online: https://www.nccn.org/professionals/physician_gls/pdf/head and neck.pdf
- Jin Y, Shi YX, Cai XY, et al. Comparison of five cisplatin-based regimens frequently used as the first-line protocols in metastatic nasopharyngeal carcinoma. J Cancer Res Clin Oncol 2012;138:1717-25. [Crossref] [PubMed]
- Zhang Y, Chen L, Hu GQ, et al. Gemcitabine and Cisplatin Induction Chemotherapy in Nasopharyngeal Carcinoma. N Engl J Med 2019;381:1124-35. [Crossref] [PubMed]
- Mok TSK, Wu YL, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet 2019;393:1819-30. [Crossref] [PubMed]
- Hodi FS, Chiarion-Sileni V, Gonzalez R, et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol 2018;19:1480-92. [Crossref] [PubMed]
- Ma BBY, Lim WT, Goh BC, et al. Antitumor Activity of Nivolumab in Recurrent and Metastatic Nasopharyngeal Carcinoma: An International, Multicenter Study of the Mayo Clinic Phase 2 Consortium (NCI-9742). J Clin Oncol 2018;36:1412-8. [Crossref] [PubMed]
- Camrelizumab (PD-1 Antibody) After Chemoradiotherapy in Locoregionally Advanced Nasopharyngeal Carcinoma (PACIFIC-NPC). 2018. Available online: https://clinicaltrials.gov/ct2/show/NCT03427827
- Co J, Mejia MB, Dizon JM. Evidence on effectiveness of intensity-modulated radiotherapy versus 2-dimensional radiotherapy in the treatment of nasopharyngeal carcinoma: Meta-analysis and a systematic review of the literature. Head Neck 2016;38:E2130-42. [Crossref] [PubMed]
- Gundem G, Van Loo P, Kremeyer B, et al. The evolutionary history of lethal metastatic prostate cancer. Nature 2015;520:353-7. [Crossref] [PubMed]
Cite this article as: Yu KKL, Agas RAF, Jacinto JCKM, Co LBA, Jacomina LE, Yap ERT, Mejia MBA. Local radiotherapy combined with systemic therapy for metastatic nasopharyngeal carcinomas at diagnosis: experience from a tertiary academic center in the Philippines. Ann Nasopharynx Cancer 2020;4:11.