
Radiotherapy dose escalation in the primary treatment of nasopharyngeal carcinoma: a systematic review and meta-analysis
Introduction
Nasopharyngeal carcinoma (NPC) is known to be highly radiosensitive, and radiotherapy (RT) remains the mainstay of treatment for non-disseminated disease (1). The treatment of NPC has undergone significant advances in the past two decades, most notably with the emergence of chemoradiation as the standard of care.
In the era of conventional/two-dimensional (2D) external beam radiotherapy (EBRT), and prior to the era of chemoradiation, dose escalation beyond 66 Gy has been shown to enhance local control. This local control benefit was particularly seen in T1/T2 primaries (2,3), with less evidence of benefit for more advanced (T3/T4) primaries (4). While the addition of brachytherapy or EBRT boost was associated with enhanced local control, it was not without additional toxicity (5-8). Whether this improved local control translated to an OS benefit and justified the increased toxicity remained unclear (9,10).
Chemoradiation or the addition of concurrent chemotherapy (CRT) to RT has led to higher tumor control rates and longer survival (11,12). The adoption of three-dimensional conformal (3DCRT) and intensity-modulated (IMRT) techniques in radiation therapy has allowed for safer delivery of higher EBRT doses up to 70 Gy to the primary tumor site (8,13). Together, these developments have resulted in a shift of the mode of treatment failure after primary therapy to mostly distant failures (12). Nevertheless, persistent local disease and local recurrences are still seen after initial therapy, which can affect patient survival and quality of life.
Local control has been shown to be directly related to the RT dose (2,8,14) and it has been suggested that a local control benefit may be derived from escalating doses beyond 70 Gy and up to 80 Gy. The development of endocavitary brachytherapy techniques that do not require soft palate dissection has allowed for further dose escalation after EBRT without increased toxicity. On the other hand, dose escalation exclusively by EBRT, either by boost or by altered fractionation, has resulted in toxicity despite the use of conformal techniques. While dose escalation beyond 70 Gy has been mostly abandoned, certain centers have continued to employ brachytherapy for this purpose.
The advent of three-dimensional image-guided planning (IGBT) and stepping-source technology in brachytherapy, stereotactic (SRS) and image-guided (IGRT) RT in more recent years warrant a second look at dose escalation. In terms of choice of modality for dose escalation, brachytherapy seems to be preferred in early T-stage NPC (T1/T2), while EBRT has been used mainly in more advanced T-stages. The optimal fractionation scheme and dose still remains to be defined. The aim of this study is to summarize the currently available evidence for RT dose escalation during the primary treatment of NPC in an effort to come up with better guideline to manage this endemic disease.
Methods
A systematic literature review was conducted using the following search engines/databases: PubMed, ASCOpubs, the Cochrane Library, and Google Scholar. The International Clinical Trials Registry Platform, CENTRAL, and clinicaltrials.gov were also searched for ongoing trials. We searched for eligible studies from the year January 01, 2000 to September 30, 2018 using the following keywords: “dose escalation” OR “boost” OR “brachytherapy” AND “nasopharyngeal cancer” OR “nasopharyngeal carcinoma” OR “NPC”. Titles of the studies from the literature search were initially screened and selected for review. Complete texts of the selected abstracts were scrutinized in detail to identify studies for inclusion based on the selection criteria. Finally, purling was done by surveying the reference lists of the identified studies. The date of the last search was on September 30, 2018. Study protocol was registered with the international prospective register of systematic reviews (PROSPERO) with ID number CRD42018096415.
Criteria for selection of studies
Types of participants
Eligible studies investigated outcomes of patients with NPC who were treated with conventionally fractionated primary RT with or without dose escalation/RT boost (boost). NPC belonging to any of the three WHO histologic subtypes were included. Studies investigating recurrent or metastatic NPC, or other histologic types of nasopharyngeal cancer (lymphoma, sarcoma, etc.) were excluded from this analysis.
Types of interventions
The primary intervention investigated was dose escalation/boost with RT during the initial therapy of NPC. RT boost was defined as the intentional addition of a RT dose to the primary tumor after initial EBRT, with cumulative biological equivalent dose (BED) exceeding 70 Gy in patients that were treated with conventional EBRT. The boost can be in the form of brachytherapy, EBRT or SRT. The boost may be done before, during or after primary RT. The comparator group was no RT boost (no boost). Concurrent chemotherapy was allowed in both groups. Response-adapted addition of RT boost after primary EBRT was also allowed. Studies which utilized non-conventional fractionation schemes for the initial EBRT like altered, accelerated or hyperfractionation were excluded to facilitate cumulative EQD2 comparisons.
Types of outcomes
Studies which reported oncologic outcomes and treatment-related toxicity of RT boost and no boost were included in this review. The primary outcomes investigated were local recurrence-free survival (LRFS) and overall survival (OS). LRFS was defined as the proportion of patients alive without a local recurrence at a specified period from the date of randomization or initiation of treatment. OS was defined as the proportion of patients alive after a specified period from the date of randomization or initiation of treatment.
Secondary outcome measures include disease-free survival (DFS), progression-free survival (PFS), and treatment-related toxicity. DFS and PFS were defined as the proportion of patients free from disease and the proportion of patients with disease but are free from any progression, respectively. Treatment-related toxicity was defined as any adverse effect directly attributable to treatment.
Types of studies
The review included two randomized controlled trials (RCT). Due to scarcity of evidence regarding the clinical question, retrospective studies were also included in this study. According to the Oxford Center for Evidence-Based Medicine, retrospective cohorts are considered as Level III evidence (15). The following types of studies were excluded in this review: case series with <10 patients per group, single-arm studies, reviews, and full articles not available in English.
Assessment of methodological quality
Critical appraisal and assessment for risk of bias was done by 3 reviewers (RA Agas, LB Co and KK Yu) using the McMaster Critical Review Form for Quantitative Studies. There were no disagreements between the reviewers regarding the eligibility of the included studies.
Data collection, synthesis, and statistical analysis
Two reviewers (LB Co, RA Agas) did independent data extraction using a tailored spreadsheet (Microsoft Excel). Disagreements with extraction were discussed at length and were ultimately resolved by a third reviewer (JC Jacinto). Data extraction included the title, author, year, study design, study population, sample size, intervention and control arms, outcome measures, and results. When appropriate, pooling of outcomes from published trial results or from data in the survival curve analysis was done. In accordance with the Cochrane Handbook for Systematic Reviews of Interventions, only outcomes from studies with similar study designs were pooled (16). Using the Review Manager Software 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark), statistical pooling was done following the Mantel-Haenszel model. The chi square statistic was used to investigate heterogeneity. Pooled data that reached an I2 of 50% or greater and/or a confidence interval <0.10 was considered to have high heterogeneity and was analyzed using the random effects model. Pooled data not meeting this cutoff were deemed to have low to moderate heterogeneity and was analyzed using the fixed effects model. Other data collected and not pooled were included in the narrative synthesis. An overall summary of evidence recommendation was done using the NHMRC of Australia Body of Evidence Framework that is composed of 5 factors: evidence base, consistency, clinical impact, generalizability, and applicability (17).
Results
Search results
The initial search yielded a total of 211 abstracts after excluding all duplicates (Figure 1). A total of abstracts were excluded due to the following reasons: prognostic studies (n=7), dosimetric studies (n=29), chemotherapy studies (n=19), applicator studies (n=3), patterns of failure (n=2), toxicity reports (n=12), quality-of-life studies (n=3), gene/genome studies (n=3), EBV/viral studies (n=8), imaging studies (n=6), treatment techniques (n=1), simultaneous integrated boost (n=26), EBRT vs. IMRT (n=1), particle therapy (n=3), recurrent disease (n=5), metastatic disease (n=1), single-arm only studies (n=24), altered fractionation (n=9), review articles (n=20), did not report outcomes (n=4), experimental/feasibility studies (n=3), case report/small series (n=5), other head & neck (n=2), and unrelated (n=5). Three studies were identified after purling (9,18,19). Full texts of 13 studies were assessed for eligibility. Three studies were excluded because the desired outcomes were not reported between comparator arms (20-22). One study had population overlap with another study (23). A total of 9 studies were found to be eligible in the final analysis (Table S1). Two RCTs were included in the review (23,24). Seven were retrospective cohorts with 988 cumulative patients eligible for statistical pooling.
Critical appraisal results
All studies were found to have sound methodological quality, stated objectives clearly, and discussed relevant background on the topic (Table S2). They had well-defined, measurable outcomes with potentially clinically meaningful results. One RCT studied brachytherapy boost in locoregionally advanced NPC (23). Another RCT explored PET-guided dose escalation via EBRT (24). Six retrospective studies utilized brachytherapy for dose escalation (2,9,18,19,25,26). One retrospective cohort studied EBRT boost (15). Patients in four studies (one EBRT, three brachytherapy) underwent dose escalation without concurrent chemotherapy (2,10,19,26).
Main results
- Randomized studies:
- LRFS: two RCTs investigated the use of boost vs. no boost in patients with stage I–IV NPC (23,24). Both studies utilized concurrent chemotherapy with the RT. Pooled 3-year LRFS for both studies was not significantly different between the boost and no boost groups with a RR of 1.04 (95% CI, 0.85–1.28, P=0.71). Heterogeneity was moderate (I2=58%) (Figure 2A);
- OS: after pooling results of OS for both RCTs, there was no significant difference between the two groups with a RR of 1.05 (95% CI, 0.93–1.18, P=0.42). Heterogeneity was low (I2=0%) (Figure 2B);
- PFS: only outcomes for 3-year PFS were available for statistical pooling. Results showed an RR of 0.97 (95% CI, 0.8–1.19, P=0.78), with high heterogeneity (I2=69%);
- DFS: pooled analysis for 3-year DFS showed a non-significant RR of 0.94 (95% CI, 0.8–1.11, P=0.48). Heterogeneity was high (I2=69%);
- Treatment-related toxicity: Both studies reported rates of late grade 3 to 4 toxicities. There were no significant differences between the dose escalation and no dose escalation groups (RR =1.00; 95% CI, 0.72–1.40, P=0.99) (Figure 2C).
- Retrospective studies:
- LRFS: 3- and 5-year LRFS rates of all seven retrospective studies were available for statistical pooling (2,9,13,18,19,25,26). All but two brachytherapy boost studies excluded T3-T4 patients (2,18,19,26). Chao et al. included T3 patients in his cohort, while Ozyar also studied patients with T3 & T4 disease. The lone study utilizing EBRT boost included only patients with primary T3 & T4 disease (15). Pooled analysis showed non-significant improvement in both 3- (Figure 3A) and 5-year LRFS (Figure 3B) between the two groups. (RR =1.04; 95% CI, 1.00–1.08, P=0.07, I2=59%) and (RR) of 1.06 (95% CI, 1.03–1.09, P=0.0003, I2=49%), respectively.
- OS: all retrospective studies reported OS outcomes, but only reported either 3- or 5-year OS. Three studies had 3-year OS and five studies had 5-year OS data available for statistical pooling (9,19,20). Both 3-year OS (Figure 3C) and 5-year OS (Figure 3D) were not significantly different with the boost vs. no boost groups (RR =1.01; 95% CI, 0.88–1.15, P=0.089, I2=84%) and (RR =1.09; 95% CI, 1.00–1.19, P=0.06, I2=77%).
- Upon subgroup analysis of four studies that only included patients not receiving concurrent chemotherapy, both 3-year LRFS (Figure 4A) and 5-year LRFS (Figure 4B) were significantly improved with the addition of boost (RR =1.03; 95% CI, 1.00–1.06, P=0.04, I2=0%) and (RR =1.05; 95% CI, 1.02–1.09, P=0.005, I2=0%) (2,10,19,26), respectively. Treatment-related toxicity: not all studies reported toxicity outcomes. Of these, cranial nerve neuropathies (grade not specified) were reported in 4 studies (2,19,25,26). There was a significantly higher rate of cranial nerve neuropathies in the group that did not receive any boost (RR =0.57; 95% CI, 0.39–0.84, P=0.005, I2=42%).
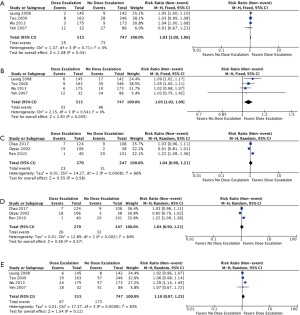 Figure 4 Pooled events from retrospective studies with or without concurrent chemotherapy. (A) 3-year LRFS without concurrent chemotherapy; (B) 5-year local recurrence-free survival without concurrent chemotherapy; (C) 3-year LRFS with concurrent chemotherapy; (D) 3-year LRFS with concurrent chemotherapy; (E) 5-year OS without concurrent chemotherapy. LRFS, local recurrence-free survival.
Figure 4 Pooled events from retrospective studies with or without concurrent chemotherapy. (A) 3-year LRFS without concurrent chemotherapy; (B) 5-year local recurrence-free survival without concurrent chemotherapy; (C) 3-year LRFS with concurrent chemotherapy; (D) 3-year LRFS with concurrent chemotherapy; (E) 5-year OS without concurrent chemotherapy. LRFS, local recurrence-free survival.
Rates of ulceration and/or necrosis of the nasopharynx were reported in 3 studies (18,19,26). A trend towards higher rates of ulceration and/or necrosis of the nasopharynx was observed in the boost group (RR =1.34; 95% CI, 0.66–2.72, P=0.41, I2=23%).
- Subgroup analysis of patients who received concurrent chemotherapy
- For the three retrospective studies with patients that received concurrent chemotherapy, both 3-year LRFS (Figure 4C) and 5-year LRFS (Figure 4D) were not significantly improved with the addition of boost (RR =1.04; 95% CI, 0.90–1.21, P=0.58, I2=86%) and (RR =1.04; 95% CI, 0.90–1.21, P=0.57, I2=84%) (9,18,25).
- Subgroup analysis of the four studies that included only patients that did not receive concurrent chemotherapy likewise showed a non-significant difference in 5-year OS between the two groups (RR =1.10; 95% CI, 0.97–1.25, P=0.012, I2=83%) (2,10,19,26).
Discussion
This review presents the current available evidence comparing dose escalation (boost) vs. no dose escalation (no boost) in the primary therapy of patients with non-metastatic NPC. Based on the National Health and Medical Research Council’s “additional levels and grades for recommendations for developers of guidelines” (Table S3), the authors recommend that the current evidence for LRFS can be trusted to guide practice in most situations. Evidence in terms of OS and treatment-related toxicity provide some support for the recommendation, but care should be taken in their application. There is limited evidence available for both PFS and DFS, which was deemed insufficient to guide current practice.
Is there an LRFS and OS benefit to dose escalation in NPC?
Overall
Our results show that dose escalation (with either brachytherapy or external beam RT) during the primary treatment of NPC does not result in significant improvement in LRFS, OS, RRFS, DFS or PFS. There was a trend towards improved 5-year LRFS after pooling all retrospective dose escalation studies.
Treated with concurrent chemoradiation? Treated with radiation alone?
Pooled analysis of the subgroup who received concurrent chemotherapy failed to demonstrate either LRFS or OS benefit (9,18,25). In the subset of patients who did not receive any concurrent chemotherapy, there was a significant 3- and 5-year LRFS benefit seen, with a trend towards improved 5-year OS (2,10,19,26). The studies included in the pooled analysis are all retrospective.
With T1/T2 disease? With T3/T4?
Pooled analysis of all patients with T1/T2 disease from retrospective studies showed improved 3- and 5-year LRFS, but no significant OS benefit (2,18,19,25,26).
Data is more limited for patients with T3/T4 disease treated with boost. Chao found no significant difference in LRFS and OS for patients with T3 disease. Rosenblatt showed that 3-year LRFS and 3-year OS were worse for patients with T3/T4 disease regardless of treatment received. Ozyar did not find any significant differences in LRFS between patients with T1/T2 and T3/T4 disease on univariate and multivariate analysis, which may be due to the limited number of patients in his analysis (9). Yeh’s study included only patients with T3/T4 disease, and failed to show significant differences in both LRFS and OS with dose escalation (10).
Treated with brachytherapy? Treated with EBRT?
Brachytherapy has traditionally been utilized as a boost in the treatment of T1/T2 disease, both in the recurrent or definitive setting. The majority of studies included in this review utilized intracavitary brachytherapy as boost, most commonly in in early stage NPC (T1/T2). Rosenblatt et al. and Ozyar et al. included both T3 and T4 patients, while Chao et al. included T3 patients (9,23,25). Both Rosenblatt and Ozyar failed to show any LRFS or OS benefit, while Chao demonstrated LRFS benefit only for patients with T1 disease. The ideal patient selection for ICBT being limited to AJCC 7E T1/T2 disease and select T3 disease with good response after EBRT.
Is there increased toxicity with dose escalation in NPC?
Overall
Pooled data for toxicity from the two randomized trials show no significant differences in Grade 3 to 4 acute & late toxicities (23,24). The most common complication noted was xerostomia. Not all of the studies reported toxicity outcomes in detail, and studies generally did not report the same toxicity outcomes. Of these, cranial nerve neuropathies (grade not specified) were reported in 4 studies (2,19,25,26). There was a significantly higher rate of cranial nerve neuropathies in the group that did not receive any boost. Rates of ulceration/necrosis of the nasopharynx were reported in 3 brachytherapy studies (18,19,26). A trend towards higher rates of ulceration/necrosis of the nasopharynx was observed in the boost group compared to the no boost group.
Treated with brachytherapy? Treated with EBRT?
Teo reported a non-significant increase in the incidence of chronic radiation nasopharyngeal ulceration/necrosis with brachytherapy. There were no complications that resulted in death or that required hospitalization (2). Ozyar did not note any BRT-related complications aside from nasal synechiae (9). While Chao saw non-significantly increased rates of CN palsy in the boost group (25). Leung had 5-year major-complication-free rates of 89.5% and 85.6% for the brachytherapy boost group and the control group, respectively (P=0.23) (26). Wu et al. noted that the incidence of complications in the EBRT + BRT group appeared to be significantly lower than those seen in the EBRT-alone group (19). The rate of nasopharyngeal ulceration or necrosis was higher in the EBRT + BRT group compared with the EBRT-alone group (2.3% vs. 0%). However, this difference was not significant (P=0.123). Ren had no significant differences in late toxicity, though more patients in EBRT group alone had NP ulceration/necrosis (19).
Wang observed no grade 4 late toxicities and no temporal lobe necrosis, and there was no significant difference in the acute radiation reactions among the three groups in their study (no boost, conventional EBRT boost and PET-guided boost) (24). Yeh had higher 5-year complication-free rates in the group that did not receive dose escalation with EBRT (10). They reported that the 5-year complication-free rates of patients receiving 70.2 and 81 Gy were 14% vs. 2% for xerostomia (P=0.0070), 50% vs. 30% for hearing impairment (P=0.0198), and 91% vs. 82% for temporal radionecrosis (P=0.0400), respectively (10).
Toxicity outcomes from some of the studies suggest that dose escalation with BRT, coupled with a lower EBRT dose, may result in fewer late complications such as trismus, neck fibrosis, cranial neuropathy, and temporal lobe necrosis. Moderate doses of BRT can result in improved local control with relatively low rates of nasopharyngeal ulceration or necrosis. However, a high total radiation dose, or large doses per fraction from EBRT or BT, could lead to perforation or ulceration of structures (i.e., soft palate, sphenoid) in close proximity to the primary site.
Modern RT techniques allow for more conformal dosimetry and employ more precise delivery techniques, while modern brachytherapy regimens use less hypofractionated techniques (3.0–4.0 Gy) and three-dimensional (CT-based) rather than two-dimensional (X-ray based) planning. These recent developments could result in better therapeutic ratio in dose-escalation.
Some of the retrospective studies included in this review are limited by small patient numbers, differing stages and histopathology of disease. None of the studies reported dose escalation specifically to the neck and/or regional disease. In spite of these study limitations, several authors have reported improvement in outcomes with dose-escalation using brachytherapy in conjunction to the standard external beam radiation. Not all studies have demonstrated a clear benefit with the addition of BRT boost. Ozyar et al. did not find a significant difference in local control rates with the addition of BRT (9). However, 32% of his cohort had T3-T4 disease. Dose coverage in such extensive disease would not have been adequately addressed with intracavitary BT. Taken together, the body of evidence would suggest that there is a local control benefit of adding brachytherapy boost after primary EBRT in T1-T2 NPC, particularly in T2 patients.
ICBT was utilized as a means of dose-escalation prior to the emergence of CCRT or IMRT. With the emergence of the latter two, there was a significant improvement of locoregional control. Dose-escalation using ICBT was abandoned by many but not all centers. IMRT was used to dose-escalate via hypofractionation (2.12–2.2 Gy per fraction) with chemotherapy. Initial toxicity limited maximum doses per fraction to 2.12 Gy.
While ICBT is currently mostly reserved for recurrences, some centers use ICBT to escalate RT doses beyond 66–70 Gy. This allows dose escalation for T1-T2 tumors without any undue increase in toxicity such as cranial neuropathy, temporal lobe necrosis or trismus. In T1N0 disease, low EBRT disease followed by boost may lower late toxicities such as CNP and trismus.
With the advent of IGBT (as with the emergence of IMRT techniques for EBRT), the role of brachytherapy in the primary treatment of NPC needs to be given a second look. The Levendag point system used for 2D planning of ICBT three-dimensional planning coupled with the stepping-source delivery now allow us to better sculpt isodose volumes and optimize treatment delivery (27).
Data is more limited for EBRT boost. Both the study by Wang and Yeh failed to show any benefit for LRFS and OS. However, in Wang’s study, there was a significant difference in LRFS and RPFS between the arms that received the lowest and highest BED (conventional chemoradiotherapy group and PET/CT-guided dose escalation chemoradiotherapy group, respectively). Both studies included only patients with T3 and T4 disease.
Currently, the use of SRT has been limited to recurrent disease, but experience in this modality seems to be increasing. Institutional single-arm reports are emerging investigating its use in the primary treatment of nasopharyngeal carcinoma (28,29). SRT has probable advantages over conventional and IMRT techniques, in the form of dose conformality and biologically equivalent doses delivered to the primary tumor.
In the current era of IMRT for the primary treatment of NPC, the majority of failures are occurring distantly. Only one study in our analysis included patients who were treated exclusively with IMRT during primary RT. IMRT allows the use of partial hypofractionation schemes during the treatment of the primary tumor, which translates to a higher effective dose received by the tumor. It remains to be seen whether further dose escalation still proffers some benefit in patients who were initially treated with IMRT. Further studies are warranted to define the role of dose escalation for these patients.
Conclusions
Dose escalation in the primary treatment of NPC does not lead to an increase in LRFS, OS, PFS or DFS. However, there seems to be a LRFS benefit with dose escalation using brachytherapy in patients with T1-T2 disease and in patients who did not receive concurrent chemotherapy. Dose escalation with brachytherapy is likewise not significantly associated with any increase in the rate of complications. Data for the efficacy and toxicity of EBRT and SRT boost is currently still lacking. More prospective studies are needed to define other subsets of patients will truly benefit from dose escalation.
Table S1
| Study | Type of study | Country (region) | Patient age | Follow-up, months | Number of patients | Staging characteristics | WHO histology | RT course | Other interventions |
|---|---|---|---|---|---|---|---|---|---|
| Rosenblatt [2014] | Randomized controlled trial | International, multi-centre; Vienna, Austria | BRT: 40+14.8; no BRT boost 43.5+13.6 | 29 [2–67] | Total: 274; BRT boost: 135; no BRT boost: 139 | BRT boost: T3–4 & N2–3: 26.7%; No BRT boost: T3–4 & N2–3: 24.5% | BRT boost: I–II: 27.4%; III: 72.6%; |
EBRT: 70 Gy in 35 fractions (parallel- opposed); |
NACT (2 cycles) cisplatin 100 mg/m2 and doxorubicin 50 mg/m2 or epirubicin 75 mg/m2 every 3 weeks; CCT weekly cisplatin 30 mg/m2 |
| Wang [2014] | Randomized controlled trial | Xuzhou, Jiangsu, China | CT-guided EBRT boost: 47 (19–67); |
36 [20–45] | Total: 67; EBRT Boost: 43; CT-guided: 22; PET-guided: 21; No EBRT boost: 24 | CT-guided EBRT boost: T1: 1, N0: 1; T2: 9, N1: 4; T3: 5, N2: 14; T4: 6, N3: 3; PET-guided EBRT boost: T1: 1, N0: 1; T2: 7, N1: 3; T3: 9, N2: 13; T4: 4, N3: 4; No EBRT boost: T1: 1, N0: 1; T2: 10, N1: 3; T3: 7, N2: 15; T4: 6, N3: 5 | CT-guided EBRT boost: WHO II: 4,WHO III: 18; PET-guided EBRT boost: WHO II: 3, WHO III: 18;no EBRT boost: WHO II: 5; WHO III: 19 | No EBRT boost: 70 Gy (IMRT) 2 Gy/fraction; |
CCT cisplatin (20 mg/m2, IV, d1–4) and docetaxel (75 mg/m2, IV, d1 and d8) administered on the 1st and 4th week of treatment; ACT: starting 4 weeks after radiotherapy of the same dose and drug regimen that ranged from 2 to 4 cycles |
| Chao [2017] | Retrospective cohort | Taipei, Taiwan | BRT boost: >50 (49%); |
63.1 [6–138] | Total: 232; BRT boost: 124; no BRT boost: 108 | BRT boost: T1: 75, N0: 38; T2: 34, N1: 43; T3: 15, N2: 35; N3: 8; no BRT boost: T1: 71, N0: 28; T2: 19, N1: 33; T3: 18, N2: 31; N3: 16 | BRT boost: WHO I: 1, WHO IIa: 15, WHO IIb: 108; no BRT boost: WHO I: 0, WHO IIa: 10, WHO IIb: 98 | IMRT, SIB, 70 Gy; |
CCT with or without ACT was used for 176 patients, including 88 with and 88 without an ICBRT boost, respectively. The most commonly used chemotherapeutic regimen was cisplatin of 40 mg/m2 weekly during the course of IMRT for 8 cycles. For Stages III and IV or other high-risk patients, standard adjuvant chemotherapy was given every 3 weeks for 3 cycles, with cisplatin 80 mg/m2/day on day 1 and fluorouracil 1,000 mg/m2 on days 1 to 4 |
| Wu [2013] | Retrospective cohort | Fujian, China | BRT boost: 44 (22–69); |
120 [5–190] | Total: 348; BRT boost: 175; no BRT boost: 173 | BRT boost: T1: 18%, N0: 33%; |
Not mentioned | EBRT: parallel opposed, 2 Gy fx; 56–60 Gy (BRT boost) 70–72 Gy (no boost); BRT: 1–4 days after EBRT; |
No standard chemotherapy protocol was used before 2000 in the author’s institution; NACT consisting of cisplatin and 5-fluorouracil was administered to 44 and 73 patients in the EBRT + BRT and EBRT groups, respectively. No concurrent chemotherapy was given |
| Ren [2010] | Retrospective cohort | Sun Yat-sen, Guangdong, China | BRT boost: >45 (43%); |
Total: 141; BRT boost: 40; no BRT boost: 101 | Only T2b included: BRT boost: N0-1: 53%, N2: 43%, N3: 5%; no BRT boost: N0-1: 57%, N2: 43%, N3: 0% | BRT boost: WHO I: 3%, WHO II/III: 97%; no boost: WHO I: 3%; WHO II/III: 97% | Bilateral opposed fields: 60 Gy (BRT boost); 68 Gy (no boost); BRT: interstitial 16 Gy | Patients with AJCC-2002 N2-3 lymph nodal involved disease (19 in the EBRT/3D-HDR-BRT group and 43 in the ERT group) received neoadjuvant, concomitant, or adjuvant chemotherapy (all cisplatin-based) | |
| Leung [2008] | Retrospective cohort | HK, China | BRT boost: 48.5 (22–78); |
98 [5–160] | Total: 287; BRT boost: 145; No BRT boost: 142 | BRT boost: T1: 79%, N0: 60%; T2a: 15%, N1: 29%; T2b: 6%, N2: 10%, N3: 1%; no BRT boost: T1: 83%, N0: 65%; T2a: 10%, N1: 21%; T2b: 7% N2: 11%; N3: 3% | BRT boost: I-II: 4%, III: 96%; no BRT boost: I-II: 8%, III: 92% | EBRT: 66 Gy (Ho’s technique); BRT: 1 week after completion of EBRT; 10 Gy in two weekly sessions for patients with T1–T2a disease and 12 Gy in two weekly sessions for patients with T2b disease | Chemotherapy not specified; 3% given in brachy group and 7% given in no boost group |
| Yeh [2007] | Retrospective cohort | Kaohsiung, Taiwan | EBRT boost: >50 (44%); no EBRT boost: >50 (44%) | 128 [81–174] | Total: 118; EBRT boost: 32; no EBRT boost: 86 | EBRT: T3: 15, N0: 8; T4: 17, N1: 11, N2: 11, N3: 2; no EBRT boost: T3: 25, N0: 20; T4: 61, N1: 28, N2: 35, N3: 3 | Not mentioned | Bilateral opposed fields; No boost patients were irradiated to a total of 70.2 Gy. In the dose escalation group, all patients were irradiated to a total of a total of 61.2 Gy via the bilateral reduced fields and three-dimensional conformal radiotherapy techniques were used to deliver an additional 19.8 Gy (total, 81 Gy). CT simulation was used | Not mentioned |
| Ozyar [2002] | Retrospective cohort | Ankara, Turkey | BRT boost: >40 (60%); |
43 [12–80] | Total: 144; BRT boost: 106; no BRT boost: 38 | BRT boost: T1: 43% N0: 24%; T2: 30% N1: 36%; T3: 12% N2: 13%; T4: 15% N3: 27%; no BRT boost: T1: 32%, N0: 18%; T2: 24%, N1: 29%; T3: 18%, N2: 37%; T4: 26%, N3: 16% | Brachy: WHO I: 6%; WHO II: 28%; WHO III: 66%; no boost: WHO I: 3%; WHO II: 16%; WHO III: 82% | EBRT: parallel opposed, 2 Gy fx; 66 Gy (median dose); BRT: immediately after EBRT 12×3 | 82 (56.9%) patients with AJCC-1988 N2 and N3 disease received neoadjuvant or concomitant cisplatin- based chemotherapy |
| Teo [2000] | Retrospective cohort | HK, China | BRT boost: >40 (60%); |
88 [5–173] | Total: 509; BRT boost: 163; No BRT boost: 346 | BRT boost: T1: 45% N0: 55%; T2: 55%, N1: 19%; N2: 21%, N3: 5%; no BRT boost: T1: 62%, N0: 34%; T2: 38%, N1: 25%, N2: 25%, N3: 15% | Not mentioned | EBRT: 60 Gy (Ho’s technique) 2–2.5 Gy/fraction; BRT: principal fractionation schemes for the ICBRT were: (I) 24 Gy/3 fractions/15 days (n=94), and (II) 18 Gy/3 fractions/15 days (n=58). The other patients (n=11) treated by ICBRT were given a dose ranging between 8 to 46 Gy delivered in 1 to 7 fractions within a period of 1 to 43 days | NACT not specified; BRT: 6%; no BRT: 16.5% |
HK, Hong Kong; RT, radiotherapy; EBRT, external beam radiotherapy; BRT, brachytherapy; CT, computed tomography; PET, positron emission tomography; T, tumor; N, node; M, metastasis; Gy, Gray; SIB, simultaneous integrated boost; IMRT, intensity-modulated radiotherapy; ICBRT, intracavitary brachytherapy; NACT, neoadjuvant chemotherapy; ACT, adjuvant chemotherapy; CCT, concurrent chemotherapy; AJCC, American Joint Committee on Cancer.
Table S2
| Study characteristic | Study | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Rosenblatt et al. (2014) | Wang et al. (2014) | Chao et al. (2017) | Wu et al. (2013) | Ren et al. (2010) | Leung et al. (2008) | Yeh et al. (2007) | Ozyar et al. (2002) | Teo (2000) | |
| Study purpose | |||||||||
| Was the purpose stated clearly | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Outline the purpose of the study. How does the study apply to your research question? | To determine whether brachytherapy boost improves outcomes in patients with advanced nasopharyngeal carcinoma treated with standard chemoradiotherapy | To determine whether PET/CT-guided radiotherapy dose escalation can improve local control while minimizing toxicity for the treatment of locally advanced nasopharyngeal carcinoma | To investigate if dose escalation using ICBT improves local control for NPC in the era of IMRT and concurrent chemoradiation | To compare efficacy and toxicity outcomes of patients with T1-T2 NPC treated with EBRT in combination with ICBT vs. a historical cohort treated with EBRT alone | To compare the results of external beam radiotherapy in combination with 3D-CT-implanted interstitial high dose rate brachytherapy (ERT/3D-HDR-BT) versus conventional external beam radiotherapy (ERT) for the treatment of stage T2b NPC | To investigate any possible therapeutic gain from dose escalation with brachytherapy for early T stage NPC | To evaluate the treatment outcomes and treatment-related complications of patients with locally advanced NPC treated with escalated radiation doses | To compare the local control and survival rates obtained with either EBRT and adjuvant HDR brachytherapy or ERT alone in patients with NPC | To study the efficacy of ICBT in early T-stage NPC |
| Literature | |||||||||
| Was relevant background literature reviewed? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Design | |||||||||
| Describe the study design. Was the study design appropriate for the study question (e.g., for the knowledge level about this issue, outcome, ethical issues)? | Randomized control trial | Randomized control trial | Retrospective cohort | Retrospective cohort | Retrospective cohort | Retrospective cohort | Retrospective cohort | Retrospective cohort | Retrospective cohort |
| Specify any biases that may have been operating and the direction of their influence on the results | Multi-institutional study with different protocols/fractionation used | Retrospective | Retrospective | Retrospective | Retrospective | Retrospective | Retrospective | Retrospective | |
| Sample | |||||||||
| No. of patients | |||||||||
| Total | 274 | 67 | 232 | 86 | 348 | 287 | 118 | 144 | 509 |
| Boost | 135 | 43 | 124 | 40 | 175 | 145 | 32 | 106 | 163 |
| No boost | 139 | 24 | 108 | 46 | 173 | 142 | 86 | 38 | 346 |
| Was the sample described in detail? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Was the sample size justified? | Yes. Sample size required not specified. Sample size required to determine number of patients needed in each arm to allow a comparison of 50% survival rate with the control treatment, to the study arm with a hypothesized survival rate of 65%, for a test of significance level of 0.05 and 80% power | No. Calculation of required sample size was not mentioned | No. This was a retrospective study | No | No | No | No | No | No |
| Sampling (who? characteristics?) | Adult patients with histopathologically proven nasopharyngeal carcinoma (WHO type I-III) and T3-T4 N0-3 or T1-T2, N2, N3 disease, according to the TNM classification of the UICC, 5th edition, were eligible for inclusion in the study. Patients had to be over 15 years of age and have an ECOG performance status of 0–2 to be eligible | Patients with previously untreated Stages III and IVA (AJCC 6th Edition) of locally advanced NPC, Karnof sky performance status >70, and good bone marrow, liver and kidney function (white blood cell count >4.0×109/L, platelets >100×109/L, albumin !30 g/L, creatinine 100 μmol/L) were enrolled in this study. Patients younger than 18, as well as those with a prior (within 5 years) or synchronous malignancy were excluded | Patients with T1–3 N0–3 M0 NPC who underwent radiation treatment with curative intent during the period August 2002 to December 2013 without gross residual primary tumors at the completion of IMRT, distant metastasis at presentation, previous history of cancers, or definitive treatment outside the hospital Patients with a pathologic diagnosis of adenoid cystic carcinoma, mucoepidermoid carcinoma, plasmacytoma or clear cell carcinoma were excluded | Patients with histologically diagnosed poorly differentiated squamous cell or undifferentiated carcinoma of the nasopharynx with early primary disease (T1-T2), with or without neck lymphadenopathy | Forty NPC patients diagnosed with stage T2b disease at the Cancer Center of Sun Yat-Sen University (Guangzhou, People’s Republic of China) between January 2004 and February 2008 were prescribed ERT with 3D-HDR-BT | Patients with early T stage NPC, after a radical course of ERT, were boosted with HDR, intracavitary remote after- loading brachytherapy during the period from 1999 to 2003 | Patients with 1992 AJCC staging system T4 classification, histology-proven nonmetastatic NPC treated at the institution between 1992 and 1995. Medical records and imaging studies were reviewed and all patients were restaged according to 2002 AJCC staging system | Between 1993 and 1999, patients with nonmetastatic NPC were treated with either ERT and BRT or ERT alone. Brachytherapy was not applied in some patients for the following reasons: (I) patients were treated before March 1994, when the high-dose-rate (HDR) BRT facility was not available; (II) less than 18 years of age; (III) treated with an accelerated hyperfractionated ERT protocol; and (IV) refused brachytherapy | All nondisseminated (M0) NPC patients with early T- stages (T1 and T2 nasal cavity tumors) presenting to the Clinical Oncology Department of the Prince of Wales Hospital, Hong Kong from 1984 to 1996 inclusive were included in the analysis |
| Describe ethics procedure. Was informed consent obtained? | Yes | Yes | No mention. Study was IRB-approved | Not mentioned | Not mentioned | Not mentioned | Yes | Not mentioned | Not mentioned |
| Outcomes | |||||||||
| Were the outcome measures reliable | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Were the outcome measures valid? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Specify the frequency of outcome measurement | Not specified | Planned patient assessments included physical examination and fiberoptic nasopharyngoscopy every 3 months, starting at 4 weeks and ending 3 years post-treatment. A contrast-enhanced CT or MRI of the head and neck is also obtained at each follow-up. After 3 years, the patients were followed yearly thereafter. Suspected recurrences were histologically proven. To assess for distant metastasis, CT of the chest and bone scan were obtained every 6 months | Not mentioned | The first clinical assessment of local disease using nasoendoscopy was typically a month after the end of RT. All patients were followed up clinically by an otolaryngologist and/or radiation oncologist every 2 months in the first year after completion of radiation, every 3 months in the second year, every 4 months in the third year, every 6 months in the fourth and fifth years, and annually thereafter. Diagnosis of local and regional recurrence was made histologically | Not mentioned | All patients were reviewed at |
After completion of radiotherapy, patients were examined at 4-week intervals until their acute radiation-related complications subsided. Patients were subsequently followed up every 2 months for the first year, every 3 months for the second year and the intervals were gradually increased to 6 months | Not mentioned | Not mentioned |
| Outcome areas; list measures used | LRFS, OS, DFS, toxicity | LPFS, RPFS, DFS, OS, toxicity | OS, LRFS | LRFS, OS, PFS, RRFS, DMFS, toxicity | LRFS, OS, DMFS, DFS, DSS, treatment-related toxicities | LR, OS, DFS | LRFS, OS, DFS, complication-free rates | LRFS, RF, DM, CSS, DFS, DMFS | Crude local failure, LRFS, DMFS, DSS, RPFS |
| Intervention | Brachytherapy boost | EBRT boost | Brachytherapy boost | Brachytherapy boost | Brachytherapy boost | Brachytherapy boost | EBRT boost | Brachytherapy boost | Brachytherapy boost |
| Intervention was described in detail | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Contamination was avoided | Yes | Yes | Not mentioned | Not mentioned | Not mentioned | Not mentioned | Not mentioned | Not mentioned | Not mentioned |
| Co-intervention was avoided | Yes | Yes | Not mentioned | Not mentioned | Not mentioned | Not mentioned | Not mentioned | Not mentioned | Not mentioned |
| Provide a short description of the intervention (focus, who delivered it, how often, setting). Could the intervention be replicated in practice? | Rotterdam Nasopharynx Applicator was used to deliver brachytherapy in the experimental study arm. Investigators were specifically trained in the use, insertion, imaging and dosimetry of the Rotterdam applicator which was used in all cases since this applicator allows for either LDR or HDR brachytherapy. The applicator was introduced transorally under topical anaesthesia, and fixed in position in the nasal cavity and nasopharynx. Then, standard after loading catheters were inserted into the applicator’s silicone tubes. In case of LDR, two Ir-192 wires with an average activity of 50–60 mCi/cm, were inserted into both after loading catheters. For HDR, the after loading catheters were connected to a remote-controlled after loader (Ir-192 microSelectron HDR). The applicator remained in place for the duration of the treatment for both LDR- and HDR endocavitary irradiation. In case of LDR, the active source was tailored to the nasopharynx proper that is to the distance between the ‘Node of Rouviere’ (at the level of the C-I vertebral body), and the pterygoid plates. The Ir-192 source was removed after a dose of 11 Gy was delivered to the nasopharynx “tumour tissue” (TT) point. For HDR, a boost dose of 9.0 Gy in 3 fractions of 3.0 Gy each was delivered with a minimum interval of 6 hours between fractions. The dose was prescribed to the “tumour tissue” (TT) points ‘Node of Rouviere (R)’ and/or ‘Nasopharynx (Na)’. The dose distribution for LDR and HDR was computed in the ‘Tumour Tissue (TT)’ points ‘Na’ and ‘R’, as well as in the critical normal tissue (NT) points representing the soft palate, base of skull, pituitary gland, optic chiasm, and retina | Areas with standardized uptake value (SUV) >2.5 were used as the GTVFor both boost groups, PTV1 received 63 Gy and PTV2 received 54 Gy, all in 1.8 Gy per fraction. In addition, the GTV received 70 Gy in 2.2 Gy per fraction in group B and the GTV received 77Gy in 2.4 Gy per fraction in group C. Radiotherapy was delivered using the SMART-IMRT technique in the dose-escalation treatment arms. For the PET/CT guided boost group, images from a diagnostic PET/CT are fused to the treatment CT to help define the GTV | The ICBT boost used the microSelectron® stepping source, remote after loading, qne HDR technique with 192-Ir as the radiation source. A pair of Nucleotron® nasopharyngeal balloon applicators with dummy seeds was inserted into bilateral nasal cavities; ICBT protocol was determined by the preference of the attending doctors. Most patients received 6Gy in 2 fractions within 1 week (range: 3–11 Gy in 1–4 fractions, within a maximum of 2 weeks after IMRT). The radiation dose of IMRT to patients with an ICBT boost was 70 Gy (range: 68–72 Gy), and to patients without an ICBT boost was also 70 Gy (range: 68–72 Gy) | For patients planned for BT, a CT or an MRI of the head and neck was ordered to assess the tumor response immediately after completion of EBRT. The interval between EBRT and BT was 1-4 days. ICBT was delivered with a microSelectron-HDR remote after loader (Nucletron, an Elekta company [Elekta AB, Stockholm, Sweden]), with an iridium-192 source strength of 37e370 GBq (1e10 Ci) as described in an earlier report (23). The Nucletron planning system (Nucletron, Elekta AB, Stock- holm, Sweden) was used, and the dose was typically prescribed at 6e13 mm from the source or 3e10-mm deep to the mucosal surface. The time dose fractionation schedule was 2.5e3 Gy twice daily, at least 6 hours apart, for 2e4 days continuously to a planned dose of 10e25 Gy; the dose of BT was used at the discretion of the attending oncologists on the basis of tumor response and the radiation dose of EBRT they had received | 3D-CT-based interstitial brachytherapy was delivered using a HDR after loading machine (microSelectron, Nucletron, Veenendaal, The Netherlands), with 192-Ir as the source and ProGuide Needle (189.601 ProGuide Needle Set 6F, sharp) used as the nasopharyngeal applicator (microSelectron, Nucletron, nylon tube technique). Patients were immobilized in the supine position with a thermoplastic mask and administered local anesthesia. The fiberoptic endo- scope was guided to the treatment sites via the inferior meatus to the treatment positions. The interstitial portion of the implant consisted of inserting 2-4 stationary ProGuide Sharp Needles in the parapharyngeal tissues of the primary tumor site followed by immobilization of the applicators. The 3D treatment plan was performed as follows: (I) ProGuide Sharp Needles with an appropriate length for interstitial treatment were placed into the treatment volume, with the needles immobilized using the button sewed into the wings of the nose; (II) CT scanning using a 0.2 cm step to obtain 0.2 cm thick slices was performed after placement of the implants and CT images were subsequently transferred to a 3D treatment planning system (PLATO PBS 14.2); (III) the target volume (i.e., the nasopharyngeal primary tumor area and the parapharyngeal involved site) and ProGuide Sharp Needles were contoured; (IV) non-parallel needles were reconstructed using the catheter reconstruction | Brachytherapy boost was given within 1 week of the completion of ERT. These patients were treated with HDR ICB using an 192Ir source (microSelectron; Nucletron, Veenendaal, The Netherlands), giving 10 Gy in two weekly sessions for patients with T1–T2a disease and 12 Gy in two weekly sessions for patients with T2b disease. This dose was prescribed at a distance of 1 cm from the center of the surface defined by the sources. The rationale for the selection of dose has been reported elsewhere. A 6-F bronchial applicator was placed inside a 3.2-mm diameter nylon tube and was used as a nasopharyngeal applicator (microSelectron nylon tube nasal technique). The applicators were positioned under topical anesthesia with fiberoptic endoscopic guidance. The three-dimensional configurations of the applicators were reconstructed using the semi-orthogonal image reconstruction method provided by the microSelectron planning system. The dose was optimized according to the geometry of the catheters using the Brachytherapy Plato Planning System (Nucletron, Veenendaal, The Netherlands). Any displacement of the catheters was verified by three-dimensional reconstruction before and after treatment. For most patients, the displacement was within 1 mm. Specific reference points were marked on the check films for calculation of doses to vital adjacent structures | In the dose escalation group, all patients were irradiated to a total of a total of 61.2 Gy via the bilateral reduced fields and three-dimensional conformal radiotherapy techniques were used to deliver an additional 19.8 Gy (total, 81 Gy). CT simulation was used. The gross extent of tumor determined from CT scan taken after 61.2 Gy rather than the pretreatment CT scan was defined as the gross tumor volume (GTV) | BRT was used after the implementation of the remote-192 controlled HDR; HDR, Nucletron-Oldeft Nucletron, Veenendaal, The Netherlands) at our department in 1994. No assessment of the primary tumor response was made at the completion of ERT, and intracavitary HDR BRT was routinely applied to all cases, except patients less than 18 years of age and patients treated with accelerated hyperfractionated ERT. BRT was delivered using a single-channel nasal applicator modified from a pediatric endotracheal tube. The tube was introduced after topical anesthesia of the nasal cavity. Either right or left nasal cavity was selected for application, according to the location or nasal cavity extension of the primary tumor or the patency of the cavity. Treatment planning in all patients was performed with optimization. The BRT dose was prescribed to a point 1 cm from the source axis, and a total dose of 12 Gy in 3 fractions was administered immediately after ERT on 3 consecutive days | ICT was performed using an in-house nasopharyngeal applicator, and the radiation dose was delivered by a HDR after loading machine (Buchler Remote After loading, Buchler GmbH, Braunschweig, Ger- many with Ir-192 source [1984 –1993], and Microselectron, Nucletron B.V. Veenendaal, The Netherlands) to a reference point at 1 cm above (and below) the midpoint of the plane of sources |
| Results | |||||||||
| Results were reported in terms of statistical significance? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Were the analysis methods appropriate? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Were the results statistically significant (P<0.05)? If not statistically significant, was the study big enough to show an important difference if it should occur? If there were multiple outcomes, was that taken into account for the statistical analysis? | Results were not statistically significant. Study was only powered to detect survival rate difference of 15%. Required sample size to detect this endpoint was not specified | Yes. But single institution pilot trial and the study may be underpowered. The data is hypothesis generating and should be further validated in a large multi-center randomized trial | Results were not statistically significant. On subgroup analysis of 75 T1 patients with ICBT boost had significantly better local control than the other 71 T1 patients without ICBT boost (98.1% vs. 85.9%, P=0.020) | Results were statistically significant for both 10-year OS and LC | Results were statistically significant for both 5-year LRFS and DFS | Results were statistically significant for 5-year LRFS, DMFS, PFS, DSS and OS | Results for all outcomes were not statistically significant | Results for all outcomes were not statistically significant | Results were statistically significant for local control & DSS |
| Was clinical importance reported? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| What was the clinical importance of the results? Were differences between groups clinically meaningful (if applicable)? | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Were dropouts reported? | Yes | Yes | Not mentioned in study | Not mentioned in study | Not mentioned in study | Not mentioned in study | Not mentioned in study | Not mentioned in study | Not mentioned in study |
| Did any participants drop out from the study? Why? (Were reasons given, and were dropouts handled appropriately?) | Yes. Data was missing on two patients; and 1 patient subsequently refused treatment in intervention arm | No. | Not mentioned in study | Not mentioned in study | Not mentioned in study | Not mentioned in study | Not mentioned in study | Not mentioned in study | Not mentioned in study |
| Conclusion and implications | |||||||||
| Conclusions were appropriate given the study methods and results | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| What did the study conclude? | The addition of a brachytherapy boost to external beam radiotherapy and chemotherapy did not improve outcome in loco-regionally advanced nasopharyngeal carcinoma | PET/CT-guided dose escalation radiotherapy is well-tolerated and appears to be superior to conventional chemoradiotherapy for locally advanced NPC | Dose escalation with ICBT can improve local control of the primary tumor for NPC patients with T1 disease treated with IMRT, even without chemotherapy | The addition of intracavitary BT to EBRT may improve the therapeutic ratio, by improving LC, particularly in T2 patients, and reducing late toxicities | The administration of interstitial 3D-HDR-BT achieved excellent local control for stage T2b NPC patients as a result of improved target coverage and conformality of the radiation dose applied | For patients who are treated with two-dimensional treatment techniques, dose escalation with brachytherapy boost improves local control and overall survival of patients with T1–T2a and possibly non-bulky T2b disease | Dose escalation to 81 Gy had no benefits on local control rate and was associated with significantly higher incidence of radiation-related xerostomia, hearing impairment, and temporal lobe necrosis. Treatment planning with fusion of CT and MRI is advised for concise target delineation | The acute and late morbidity of adjuvant HDR BRT is acceptable with our treatment scheme, but authors did not find any local control difference between our patients treated with adjuvant BRT after ERT and ERT alone | ICT significantly enhanced ultimate local control and avoided the necessity for morbid salvage treatments in early T-stage (T1/T2 nasal infiltration) NPC. The slight increase in chronic radiation ulceration/necrosis after ICT was acceptable with mild and manageable symptoms. Other late complications were not increased. A significant dose–tumor-control relationship exists above the conventional tumoricidal dose level |
| What are the implications of these results for practice? What were the limitations or biases in the study? | Results were inferior to studies reported in other series from Asia. Included advanced T-stage NPC patients in study. Multi-institutional study with different protocols/fractionation used. Only 80% of patients underwent treatment in intervention group. T3 & T4 patients included. Sample size required was not specified in study | Study was a single institution pilot trial and may be underpowered. Required sample size was not calculated. The data is hypothesis generating and should be further validated in a large multi-center randomized trial | Retrospective | Retrospective. Some variation in the external beam and BT doses | Retrospective | Retrospective | Retrospective. Dose escalation was done via 3DCRT | Retrospective. Included T3 & T4 patients in study | Retrospective. Some variation in the external beam and BRT doses |
NPC, nasopharyngeal carcinoma; RT, radiotherapy; BRT, brachytherapy; EBRT, external beam radiotherapy; IMRT, intensity-modulated radiotherapy; LR, local recurrence; LRFS, local recurrence free survival; OS, overall survival; DSS, disease specific survival; DFS, disease free survival; PFS, Progression Free Survival; DM, Distant Metastasis; DMFS, Distant Metastasis Free Survival; LDR, low dose-rate; HDR, high dose-rate; ECOG, Eastern Cooperative Oncology Group; UICC, Union for International Cancer Control; AJCC, American Joint Committee on Cancer.
Table S3
| Components | Grade | Comments |
|---|---|---|
| Evidence base | ||
| Local recurrence/local recurrence free survival | B | Two randomized controlled trials and seven retrospective studies with low to moderate risk of bias were included in the review |
| All data on the subgroup analysis without concurrent chemotherapy were from four retrospective studies | ||
| Data on EBRT boost is available only from one randomized controlled trial and one retrospective study | ||
| Overall survival | B | Two randomized controlled trials and six retrospective studies with low to moderate risk of bias were included in the review. Not all retrospective studies reported outcomes that were eligible for statistical pooling |
| All data on the subgroup analysis without concurrent chemotherapy were from four retrospective studies | ||
| Data on EBRT boost is available only from one randomized controlled trial and one retrospective study | ||
| Disease free survival | C | Two randomized controlled trials with low risk of bias were included in the review. One randomized trial had a small population |
| Progression free survival | C | Two randomized controlled trials with low risk of bias were included in the review. One randomized trial had a small population |
| Treatment related toxicities | C | Two randomized controlled trials and four retrospective studies with low to moderate risk of bias were included in the review |
| Not all retrospective studies reported outcomes that were eligible for statistical pooling | ||
| Consistency | ||
| Local recurrence/local recurrence free survival | B | Both randomized controlled trials failed to show significant benefit in local recurrence-free survival, although the lack of benefit in one study may be due to small sample size |
| Almost all of the retrospective studies that demonstrated a LRFS benefit involved patients with T1-T2 disease. Studies that included T3-T4 patients failed to show any significant LRFS benefit and showed a trend with worse outcomes | ||
| Overall survival | C | Both randomized controlled trials failed to show significant benefit in local recurrence-free survival, although the lack of OS benefit in one study may be due to small sample size |
| Some of the retrospective studies that demonstrated an OS benefit in involved patients with T1-T2 disease, while the others failed to demonstrate a survival benefit. Studies that included T3-T4 patients failed to show any significant OS benefit and showed a trend with worse outcomes | ||
| Disease free survival | C | Both randomized controlled trials failed to show significant benefit in local recurrence-free survival, although the lack of OS benefit in one study may be due to small sample size |
| Progression free survival | C | Both randomized controlled trials failed to show significant benefit in local recurrence-free survival, although the lack of OS benefit in one study may be due to small sample size |
| Treatment related toxicity | B | Both randomized controlled trials did not demonstrate a difference in treatment-related toxicity between the two groups. Not all retrospective studies reported treatment-related toxicity that were eligible for statistical pooling |
| Clinical impact | ||
| Local recurrence/local recurrence free survival | A | Dose escalation with brachytherapy for T1/T2 disease results in a LRFS benefit. Brachytherapy boost in T3/T4 disease may result in worse outcomes, although data available is scant |
| Data on EBRT boost is available only from one randomized controlled trial and one retrospective study | ||
| Pooled data from studies including only patients who did not receive concurrent chemotherapy showed LRFS benefit with dose escalation | ||
| Overall survival | C | Dose escalation did not result in significant OS benefit after pooling of eligible studies. Some studies reported OS benefit with the addition of brachytherapy boost in T1/T2 disease |
| Disease free survival | D | The absence of a significant benefit in disease free survival may indicate that systemic failure remains a significant problem even in patients with locally controlled disease |
| Progression free survival | D | The absence of a significant benefit in progression free survival may indicate that systemic failure remains a significant problem even in patients with locally controlled disease |
| Treatment related toxicity | C | There was no significant increase in toxicity from dose escalation with brachytherapy. Toxicity data for dose escalation using EBRT or SRT is still inadequate |
| Generalizability | ||
| Local recurrence/local recurrence free survival | B | The results are more generalizable to patients with early primary stage (T1-T2) NPC compared to those with advanced (T3-T4) disease |
| Overall survival | B | The population studied in the body of evidence is similar to the target population |
| Disease free survival | B | |
| Progression free survival | B | |
| Treatment related toxicity | B | |
| Applicability | ||
| Local recurrence/local recurrence free survival | C | Dose escalation is most applicable at the few centers with brachytherapy expertise in the local setting |
| Overall survival | C | |
| Disease free survival | C | |
| Progression free survival | C | |
| Treatment related toxicity | C | |
| Overall | ||
| Local recurrence/local recurrence free survival | B | The body of evidence can be trusted to guide practice in most situations |
| Overall survival | B | The body of evidence provides some support for the recommendations but care should be taken in their application |
| Disease free survival | C | |
| Progression free survival | C | |
| Treatment related toxicity | C | |
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/anpc.2019.04.01). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ho JH. An epidemiologic and clinical study of nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 1978;4:182-98. [Crossref] [PubMed]
- Teo PM, Leung SF, Lee WY, et al. Intracavitary brachytherapy significantly enhances local control of early T-stage nasopharyngeal carcinoma: the existence of a dose-tumor-control relationship above conventional tumoricidal dose. Int J Radiat Oncol Biol Phys 2000;46:445-58. [Crossref] [PubMed]
- Leung TW, Tung SY, Wong VY, et al. Nasopharyngeal intracavitary brachytherapy: the controversy of T2b disease. Cancer 2005;104:1648-55. [Crossref] [PubMed]
- Tate DJ, Adler JR, Chang SD, et al. Stereotactic radiosurgical boost following radiotherapy in primary nasopharyngeal carcinoma: impact on local control. Int J Radiat Oncol Biol Phys 1999;45:915-21. [Crossref] [PubMed]
- Wang CC. Improved local control of nasopharyngeal carcinoma after intracavitary brachytherapy boost. Am J Clin Oncol 1991;14:5-8. [Crossref] [PubMed]
- Vikram B, Mishra S. Permanent iodine-125 (I-125) boost implants after external radiation therapy in nasopharyngeal cancer. Int J Radiat Oncol Biol Phys 1994;28:699-701. [Crossref] [PubMed]
- Chang JT, See LC, Tang SG, et al. The role of brachytherapy in early-stage nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 1996;36:1019-24. [Crossref] [PubMed]
- Levendag PC, Schmitz PI, Jansen PP, et al. Fractionated high-dose-rate brachytherapy in primary carcinoma of the nasopharynx. J Clin Oncol 1998;16:2213-20. [Crossref] [PubMed]
- Ozyar E, Yildz F, Akyol FH, et al. Adjuvant high-dose-rate brachytherapy after external beam radiotherapy in nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 2002;52:101-8. [Crossref] [PubMed]
- Yeh SA, Huang YJ. Dose escalation for patients with locally advanced nasopharyngeal carcinoma treated with radiotherapy alone. Am J Clin Oncol 2007;30:401-5. [Crossref] [PubMed]
- Al-Sarraf M, LeBlanc M, Giri PG, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized Intergroup study 0099. J Clin Oncol 1998;16:1310-7. [Crossref] [PubMed]
- Blanchard P, Lee A, Marguet S, et al. Chemotherapy and radiotherapy in nasopharyngeal carcinoma: an update of the MAC-NPC meta-analysis. Lancet Oncol 2015;16:645-55. [Crossref] [PubMed]
- Hsiung CY, Yorke ED, Chui CS, et al. Intensity-modulated radiotherapy versus conventional three-dimensional conformal radiotherapy for boost or salvage treatment of nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 2002;53:638-47. [Crossref] [PubMed]
- Yan JH, Qin DX, Hu YH, et al. Management of local residual primary lesion of nasopharyngeal carcinoma (NPC): are higher doses beneficial? Int J Radiat Oncol Biol Phys 1989;16:1465-9. [Crossref] [PubMed]
- Centre for Evidence-Based Medicine: Levels of evidence. Available online: https://www.cebm.net/2009/06/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/
- Higgins J. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011).
- National Health and Medical Research Council: NHRMC additional levels of evidence and grades for recommendation for developers of guidelines.
- Ren YF, Gao YH, Cao XP, et al. 3D-CT implanted interstitial brachytherapy for T2b nasopharyngeal carcinoma. Radiat Oncol 2010;5:113. [Crossref] [PubMed]
- Wu J, Guo Q, Lu JJ, et al. Addition of intracavitary brachytherapy to external beam radiation therapy for T1-T2 nasopharyngeal carcinoma. Brachytherapy 2013;12:479-86. [Crossref] [PubMed]
- Syed AM, Puthawala AA, Damore SJ, et al. Brachytherapy for primary and recurrent nasopharyngeal carcinoma: 20 years’ experience at Long Beach Memorial. Int J Radiat Oncol Biol Phys 2000;47:1311-21. [Crossref] [PubMed]
- Lu JJ, Shakespeare TP, Tan LK, et al. Adjuvant fractionated high-dose-rate intracavitary brachytherapy after external beam radiotherapy in Tl and T2 nasopharyngeal carcinoma. Head Neck 2004;26:389-95. [Crossref] [PubMed]
- Lee N, Hoffman R, Phillips TL, et al. Managing nasopharyngeal carcinoma with intracavitary brachytherapy: one institution’s 45-year experience. Brachytherapy 2002;1:74-82. [Crossref] [PubMed]
- Rosenblatt E, Abdel-Wahab M, El-Gantiry M, et al. Brachytherapy boost in loco-regionally advanced nasopharyngeal carcinoma: a prospective randomized trial of the International Atomic Energy Agency. Radiat Oncol 2014;9:67. [Crossref] [PubMed]
- Wang J, Zheng J, Tang T, et al. A Randomized Pilot Trial Comparing Position Emission Tomography (PET)-Guided Dose Escalation Radiotherapy to Conventional Radiotherapy in Chemoradiotherapy Treatment of Locally Advanced Nasopharyngeal Carcinoma. PloS One 2015;10:e0124018 [Crossref] [PubMed]
- Chao HL, Liu SC, Tsao CC, et al. Dose escalation via brachytherapy boost for nasopharyngeal carcinoma in the era of intensity-modulated radiation therapy and combined chemotherapy. J Radiat Res (Tokyo) 2017;58:654-60. [Crossref] [PubMed]
- Leung TW, Wong VY, Sze WK, et al. High-dose-rate intracavitary brachytherapy boost for early T stage nasopharyngeal carcinoma{private}. Int J Radiat Oncol Biol Phys 2008;70:361-7. [Crossref] [PubMed]
- Bacorro WR, Agas RA, Cabrera SM, et al. A novel applicator design for intracavitary brachytherapy of the nasopharynx: Simulated reconstruction, image-guided adaptive brachytherapy planning, and dosimetry. Brachytherapy 2018;17:709-17. [Crossref] [PubMed]
- Wu SX, Chua DT, Deng ML, et al. Outcome of fractionated stereotactic radiotherapy for 90 patients with locally persistent and recurrent nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 2007;69:761-9. [Crossref] [PubMed]
- Liu F, Xiao JP, Xu GZ, et al. Fractionated stereotactic radiotherapy for 136 patients with locally residual nasopharyngeal carcinoma. Radiat Oncol 2013;8:157. [Crossref] [PubMed]
Cite this article as: Co LBA, Agas RAF, Jacinto JKM, Yu KKL, Mejia MA, Bacorro WR. Radiotherapy dose escalation in the primary treatment of nasopharyngeal carcinoma: a systematic review and meta-analysis. Ann Nasopharynx Cancer 2019;3:1.






