
Proton/heavy ion therapy in salvage of locally recurrent nasopharyngeal carcinoma
Introduction
The success rate of nasopharyngeal carcinoma (NPC) treatment has vastly improved over the past 20 years thanks to the advancement in both diagnostic and treatment technique and technology (1). Modern imaging can provide an accurate anatomical picture showing the extent of tumor invasion. The advancement of systemic treatment and its combination with radiotherapy has also contributed to the improvement in the treatment outcome. Most importantly the development of intensity modulated radiotherapy (IMRT) has made it possible to sculpt the high radiation dose distribution to the extensive and highly irregular target volume while sparing the neighboring critical structures. A curative dose of 70 Gy or more can now be readily delivered to the target volume without exceeding the maximum tolerable dose (MTD) limit of the surrounding critical volumes like the brain stem, spinal cord, optic nerves and chiasm, etc.
Unfortunately, local recurrence still occurs in 5–10% of the patients despite the advanced primary treatment (2,3). In particular the local recurrence rate can amount to 26% in 5 years for T4 diseases (4,5).
For early local recurrent NPC, surgery remains the favorable modality for salvage treatment (6,7). Radiotherapy is difficult to re-apply due to the high radiation dose already taken by the critical structures during the primary treatment. Re-irradiation with salvage intent has been associated with severe and sometimes fatal late complications (8-10). It seems we might be approaching the limit of using X-ray as a mean to deliver a desirable dose distribution for the salvage of recurrent NPC. The potential of further improving the treatment outcome by the very promising physical dose distribution of high energy proton or heavy ion beams therefore make them a very attractive alternative for salvage treatment of advanced local recurrence of NPC (6).
Proton/heavy-ion facilities worldwide
The use of protons for radiotherapy was first suggested by Robert Wilson in 1946. With advancement in technology and increased interest in achieving a dose distribution not achievable with X-ray IMRT, the number of proton/heavy-ion therapy facilities has increased dramatically over the past decade. According to Proton Therapy Co-Operative Group (11), there are currently more than 100 particle therapy centers operating around the world, and majority of them are performing proton therapy. Around 10% of them are using carbon ions. Up to 2018, the number of patients who have received proton/carbon-ion therapy has amount to around 220,000, of which around 190,000 were treated with protons. Some National and professional organizations have established guidelines regarding the indication of proton therapy. For the re-irradiation of recurrent NPC, while clinical evidences are still being gathered, re-irradiation is being gradually accepted as an indication for proton therapy. ASTRO Proton Beam Therapy Model Policy (12) stated that re-irradiation cases where cumulative criterial structure dose would exceed tolerance dose warrants the referral for proton therapy, the latest National Comprehensive Cancer Network (NCCN) guidelines for head and neck cancers (13) also stated that proton beam therapy “may be used for reirradiation when normal tissue constraints cannot be met by photon-based therapy”. Carbon ion radiation therapy (CIRT) is less widely available and is still considered “experimental” for many tumor sites, but guidelines of clinical indication are being established (14,15).
Physical dose characteristics
The most prominent difference between high energy proton/heavy-ion beam and conventional MV X-ray is in their depth dose curves as shown in Figure 1A. Proton/heavy-ion beam delivers majority of its dose at the end of range (the Bragg peak) and almost no dose afterward, allowing a much better normal tissue sparing before and beyond the tumor region. The Bragg peak is usually too sharp for treatment and different techniques have been developed to stack the Bragg Peaks at different depths together to produce a spread-out Bragg peak (SOBP) to cover a sizable tumor (Figure 1B). The Bragg peak produced by carbon ion is even sharper than proton and might lead to a SOBP of higher entrance dose (16) (Figure 1C). A more pronounced “tail” is also observed after the carbon ion range due to the nuclear interactions leading to fragmentation of carbon ions into lighter particles (17).
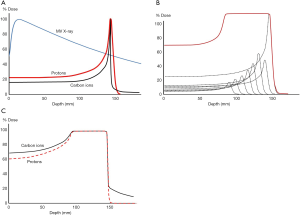
The penumbra of the high energy proton beam can be made narrower than X-ray for up to around 15 cm deep, the penumbra of carbon ion beam is even sharper due to less scattering. There is less suppression of Bragg peak with small field size and this facilitates better dose control in spot scanning (more on spot scanning in the later section) using narrow pencil beams. Chu (18) showed that with a 1 cm diameter aperture, the ratio of Bragg peak to entrance dose of a 150 MeV proton Bragg peak is reduced to half while such collimation had negligible effect on carbon ions reaching similar range.
Biological considerations
It is generally believed that recurrent NPC are more radiation-resistant (6), for which high linear energy transfer (LET) radiations like proton/heavy-ion beams are considered more effective as they inflict more direct double-strands breaking DNA damage (17-19). LET also increases towards the end of particle range and a higher LET (up to around 200 keV/µm) yields a higher relative biological effectiveness (RBE). Currently most proton therapy treatments and studies assume a fixed RBE of 1.1 and the RBE of carbon ions has been estimated to be between 2 and 5 depending on the particle energy (depth) and tissue type (20). The biological dose of proton/heavy-ion therapy is usually expressed as “Gy equivalent” (GyE) after taking the RBE into account. With increasing amount of studies and clinical experience, many are now questioning the validity of a fixed RBE for proton beam (21,22). The complex variation of RBE means one must be very cautious when deciding the dose constraints of organ at risk (OAR) and even beam directions (20) (avoid putting critical structures at the end of range) when using these high LET radiations.
Delivery of proton/heavy-ion therapy
As the Bragg peak is usually too sharp for treating a sizable tumor, two major techniques have been developed to deliver a uniform dose to the whole of the target volume. They are usually known as passive scattering and spot scanning. There are other derivatives from these methods but their principles are basically the same.
Passive scattering
In a passive scattering system, a board beam is produced by passing the narrow particle beam through scatterers. Figure 2A shows the typical components found in the nozzle in a passive scattering system. The two scatterers are used to board the narrow particle beam. A range-modulating wheel or a ridge filter “pulls” the Bragg peak of the particle beams to different depths and create a SOBP of desired length. Two sets of “wobbling” magnets can also be used to produce a board beam by scanning the narrow beam over a pre-determined area. This reduces the amount of energy spread but also reduces the speed of forming a complete board beam.
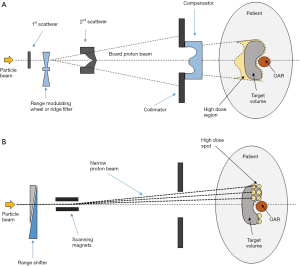
The range modulating wheel or ridge filter is chosen to create a SOBP for the target extent. The distal range of the SOBP is usually positioned to the distal edge of the target volume with margin for uncertainties. The collimator shapes the field and reduces the penumbra. The compensator modulates the range of different part of the board beam. There are always some high-dose regions outside the tumor where its thickness is shorter than this maximum extent (in this case proximal to the target). The target dose from the SOBP is designed to be uniform and not modulable.
Spot scanning
In contrast to using a broadened particle beam and SOBP, the spot-scanning technique scans the narrow pencil beam 3-dimensionally over the target volume to achieve the required dose level and uniformity. A typical beam line is shown in Figure 2B. The variable range shifter controls the range of the narrow particle beam and the scanning magnets control its direction. The Bragg peak can be placed anywhere inside the patient for any amount of time as desired like. It is like filling up the target volume with spots of high dose (hence spot-scanning). Spot scanning requires more sophisticated delivery system and may suffer from interplay effect due to patient movement. However, it allows more specific control on dose distribution. No beam-specific compensator is required and the high dose region outside the tumor for each beam due to a fixed length SOBP can be avoided.
In addition to delivering a uniform dose with every single beam [single field optimization (SFO)], spot scanning sequence can be made to deliver non-uniform dose distribution per beam that can complement each other when multiple beams are used [multiple field optimization (MFO)]. MFO is analog to IMRT in X-ray radiotherapy and is also referred to as intensity modulated proton therapy (IMPT) (for proton beams). SFO is more resilient to patient movement and setup error (23), but MFO may be preferable when highly modulated dose distribution is required, and especially when patient motion is not a problem with that treatment site.
The uncertainties in proton/heavy-ion therapy
Planning target volume (PTV) as suggested by ICRU reports 50 has long been used to account for radiotherapy setup uncertainty, including the uncertainty introduced during planning image acquisition and any setup uncertainty in the delivery sessions. Such use of PTV could be seen in earlier reports on proton/heavy-ion therapy treatment (19,24). Compared with X-ray, proton/heavy-ion beam is very sensitive to range error, which can lead to significant over or under dosage (Figure 3). The concept of PTV margin to account for geometrical uncertainty may not be adequate (25,26) and the dose delivery uncertainty is now often considered on a beam-by-beam (or spot-by-spot) basis.
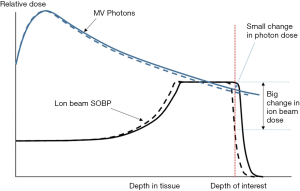
The uncertainties come from many sources. Firstly, treatment plans are designed based on the patient anatomy information obtained from CT images, which are produced with kilovoltage X-ray. The derivation of the proton/heavy-ion stopping power from CT number in various types of tissue usually found in human body (27) is not perfect and can induce error as large as 5 mm in a 10 cm particle range.
The error in range can also attribute to any misalignment of patient position and the change of patient anatomy due to respiration motion or during the course of treatment that can span over many weeks. Even variation in body cavity filling can lead to significant change in range and the resultant dose distribution inside the patient.
A typical setup uncertainty used in head and neck treatment is around 3 mm and the range uncertainty around 3% (28-31) for each beam direction. To account for such uncertainties one can design a treatment plan so that the optimized dose distribution is acceptable under the worst case scenario (32,33), or incorporate the uncertainties into the optimization process to produce a plan that is resilient to setup and range errors (25,34,35). Nevertheless, the allowance for such uncertainties may significantly impact the dose-sculpting capability of using proton/heavy-ion beams when critical structures are very close to the target volume.
Treatment plan comparison with IMRT and patient selection
While the energy deposition characteristics of proton/heave-ion beam are superior to that of X-ray, the actual benefits need to be studied and quantified for different body sites in different clinical situations. Many plan comparisons have been made for the head and neck regions (36-39). A report by Barten et al. (23) comparing photon volumetric modulated arc therapy (VMAT) and spot-scanning proton therapy for head and neck cancer with robustness taken into account found that MFO (or IMPT) resulted in better OAR sparing, but was less robust than SFO. SFO however could only marginally improve OAR sparing over VMAT. For recurrent NPC, Liu et al. (40) compared IMRT with proton therapy plans for a group of 7 patients. Only three coplanar proton beams in passive scattering mode were used against a 9-field IMRT. 66 and 62.7 GyE were planned for the gross tumour volume (GTV) and PTV respectively. Optimal and similar target coverage could be achieved by both IMRT and proton therapy. However, the proton plans could achieve a much lower dose to the critical structures. The median maximum brainstem dose was 27.9 GyE with protons versus 42.5 GyE with IMRT (P<0.01). For spinal cord proton plans only gave a maximum dose of 8.4 GyE (median among group) while IMRT delivered 22.9 GyE (P<0.004). Wang et al. (41) compared 5- to 9-field IMRT with 2- to 3-field IMCT plans on 10 locally recurrent NPC patients. PTV was created for treatment planning with a prescribed target dose ranged from 57 to 60 GyE. They found no difference in CTV dose coverage or dose conformity. The maximum dose to brain stem (35.7 GyE vs. 41.5 Gy, P=0.022), spinal cord (9.4 GyE vs. 19.7 Gy, P=0.022) and V30 of parotid glands (0 vs. 2.6 cc, P=0.028) were significantly lower with IMCT. In an unpublished study in our institution, we compared proton plans (3–4 fields) with 9-field IMRT for 20 recurrent NPC patients who had already received 70 Gy in their primary treatments. Robust optimization was employed with 3% range uncertainty and 3 mm alignment uncertainty. 60 GyE was to be given to the target volume and the cumulative dose limits of OARs were set at 130% of dose constraints of OARs of primary course. The V100% of GTV and clinical target volume (CTV) were more than 10% higher with IMPT (P<0.05). Significant sparing was also achieved with IMPT delivering significantly lower maximum dose to spinal cord, brain stem, optic chiasm optic nerve and temporal lobe. However, there were hot spots within the target volumes leading to significant increase in doses to the carotid artery and nasopharyngeal mucosa.
In addition to studying the dosimetric advantages for specific groups of patients, planning comparison are also being applied to select the right patients for proton/heavy-ion therapy due to their high cost and limited availability. Usually two treatment plans, IMRT and proton/heavy-ions, are produced and compared for the same patient. Only when substantial dosimetric superiority exists that the patient will be selected for proton/heavy-ion therapy. Delaney et al. (42) demonstrated the feasibility of using a knowledge-based planning solution to speed up the comparison process in which the knowledge-based planning solution successfully identified 4 out of 5 patients that would receive at least 6 Gy difference in mean dose to the combined swallowing and salivary structures.
Another approach is to compare the predicted clinical outcome based on clinical models. Langendijk et al. (43) reported on a selection method based on the reduction of complication probability. The model was accepted by the Dutch health authorities to select patients for proton therapy. Apart from the treatment plan performance, other criteria not directly related to the treatment plan itself could also be considered. In particular, patients with extensive metastases or with a short life expectancy due to natural course may not benefit from the OAR sparing capability of proton therapy (44). Model considering the complication probability, quality of life, life expectancy, etc., are being developed to assist in the selection process (45,46). It is yet to see if such patient selection method will be applicable for recurrent NPC patients.
The dose constraints for locally recurrent NPC
It is a general consensus that 60 Gy or higher is required for treating recurrent NPC (8,47,48). The dose constraints to the critical structures, however, show a large variation due to the consideration on repair after primary radiotherapy treatment. In photon therapy, Chan et al. (49) reported the use of maximum tolerable lifetime physical dose of 130% of the single-course limit for recurrent NPC treatment, which was found to be a bit conservative from the outcome analysis. Lee et al. (50) suggested using a maximum lifetime BED of 130% of that for primary treatment, and their lifetime BED (with α/β ratio =2.5 Gy) of spinal cord, brainstem and optic chiasm are 100, 130 and 130 Gy2.5 respectively. Partial recovery from the first course of treatment by approximately 50% (provided that the first course was delivered more than 1 year ago) is also commonly used (51,52).
In the published series of proton/heavy-ion therapy treatment on recurrent NPC, Dionisi et al. (53) used a maximum 64 Gy for brain stem and assumed a 30–50% brain stem recovery. They did not assume any recovery on optical structure and applied a maximum cumulative dose of 64 Gy, as well as a 120 Gy cumulative dose limit for carotid artery. Hu et al. (54) assumed OAR’s had a 70% recovery from the primary radiotherapy treatment. The dose constraints used were optic nerve (D20% <30 GyE), brain stem (max dose <45 GyE), spinal cord (max dose <30 GyE) and temporal lobes (V40 <7.66 cc, V50 <4.66 cc), the rest followed the TD5/5 (5% complication in 5 years) described by Emami et al. (55). The historical series by Lin et al. (19) did not described their dose constraints for the re-irradiation of critical structures. They did report that, while the maximum dose to the brain stem ranged from 1.8 to 20 GyE, the mean D90%, D50% and D10% was only 0.5, 1.2 and 2.2 GyE respectively. The maximum dose to optic chiasm only ranged from 0 to 3.8 GyE. While the maximum dose to the spinal cord was up to 22 GyE, the mean D90%, D50% and D10% was only 0, 1.4 and 3.7 GyE respectively. There were some infrequent side effects but no central nervous system complication.
Clinical outcome analysis
There have been many reports on the application of proton and heavy ion therapy on head and neck cancers. For example, Blanchard et al. (56) compared the clinical outcome of IMPT vs. IMRT on patient with oropharynx but found no significant difference in overall survival (OS) or progression free survival (PFS). The use of IMPT did result in a slightly lower gastrostomy tube (G-tube) rate and lesser weight loss. Holliday et al. (57) compared IMRT with IMPT on treating two matched groups of NPC patients and found that IMPT could significantly lower the mean doses to the oral cavity, brainstem, whole brain and mandible, and the lower mean dose to oral cavity was associated with a lower G-tube rate. The IMPT group also experienced significantly less grade 3 acute toxicities (P=0.015) (there being no grade 4 or 5 toxicities).
A few have reported on the use of proton therapy on recurrent head and neck cancers. Jensen et al. (58) reported on the outcome of treating 16 patients (2 were NPC) with scanning carbon ions and proton beams, McDonald et al. (30) looked at the proton therapy treatment of recurrent and second primary head and neck cancer in 61 patients (including 7 NPC patients). Phan et al. (28) and Romesser et al. (29) both reported their outcomes of irradiating recurrent head and neck cancers with proton therapy although in each group only a few patients were suffering from recurrent NPC.
The use of proton/heavy-ions in treating recurrent NPC dated back to the last millennium. Feehan et al. (24) reported on treating 11 recurrent locally advanced NPC patients with He and Neon ions, sometimes as a combination with photon therapy. The median target dose was 50 GyE (31.8–62.3 GyE). With a median follow up period of 28.1 months, the OS was 59% at 3 years and 31% at 5 years. The local control (LC) at the time of analysis was 45%.
Another early series by Lin et al. (19) reported on the treatment outcome of re-irradiation of NPC patient with proton therapy. While the OS and LC were only 50% at 24 months, those who could get an “optimal” tumor dose coverage had a significantly higher survival (83% vs. 17%, P=0.006). Their result demonstrated the utmost importance of a superior dose distribution in getting a good outcome in salvaging recurrent NPC.
Majority of the historical studies on treating recurrent NPC with proton/heavy ion therapy employed the passive scattering technique as spot-scanning has only become more available in the last decade (59). Recently, Dionisi et al. (53) reported the clinical outcome of using spot-scanning proton therapy in treating 17 recurrent NPC patients. The previous photon plan for the primary NPC treatment were obtained and used for cumulative dose analysis to ensure the critical structures were not overdosed. A median of 60 GyE (30.6–66 GyE) was given to the target volumes. For treatments planned with SFO technique, a PTV with 4mm margin was used. For treatment planned with MFO technique, robust optimization incorporating a 3.5% range uncertainty was applied for a PTV created with 3 mm margin for setup uncertainty. A single RBE value of 1.1 was applied in their treatment. The OS and LC at 18 months were 54.4% and 66.6% respectively. They increased to 59.3% and 72.9% if patients treated with palliative intent were excluded. The result is not much different from that reported by the Loma Linda group (19). However, unlike the Loma Linda group (19), they did not find any correlation between tumor coverage and treatment outcome.
Initial clinical outcome from salvage treatment of recurrent NPC using carbon ion was reported by Hu et al. (54). Intensity modulated carbon ion therapy (IMCT) was performed and 75 patients were included. The IMCT dose of 50–66 GyE, delivered with 2 to 3 carbon ion beams, was slightly lower than that in Dionisi et al. (53). A PTV created using 3–6 mm margin from the CTV was used. The treatment gap from the primary radiotherapy was at least 6 months and they assumed a 70% recovery from the previous IMRT treatment. The OS at 1 year was 98.1%, and the local relapse free survival was 86.6%. While no patients developed grade 2 or above acute toxicities, grade 3 or higher late toxicities were observed with one patient died of massive hemorrhage. The median follow-up time was 15.4 months and at the time of analysis, 21 and 1 patients had developed local and regional failures respectively. As a comparison, most historical series on treating recurrent NPC with IMRT led to higher than 50% serious toxicity that developed at a median time of around 6 months after treatment (60). In a recent study (10) for recurrent NPC patients, the 5-year OS and local failure free survival was around 41% and 72% respectively, accompanied by a 33% grade 5 toxicity rate. The improvement in long term clinical outcomes using proton/heavy-ion therapy over IMRT for this group of recurrent NPC patients are yet to be determined.
A summary of selected clinical outcomes for treatment of recurrent NPC with proton/heavy-ion therapy is in Table 1. Although the treatment outcomes are considerably better than with IMRT using X-ray, it appears that, despite the advancement in knowledge, technology and technique in both diagnosis and radiotherapy treatment, the treatment outcome of recurrent NPC with proton/heavy-ion therapy has not significantly improved over the past 30 years. Perhaps the abutting critical structures are significantly hindering the target dose coverage with our current ability to control the delivery uncertainty. As Mahajan (44) pointed out, when the critical structures are close to or even unavoidably located inside the high dose region, the dose-sculpting capability of proton/heavy-ion therapy might not be of much help. It may also be due to the huge success in treating primary NPC with IMRT in combination of chemotherapy, that the NPC that recurs today are the most difficult ones and the most radiation-resistant (6). Locally advanced recurrent NPC remains a very challenging disease to treat and more efforts are still required to refine the application of proton/heavy-ion therapy for this group of patients.
Table 1
| Report | Patient number | Particle | Target dose, GyE | Overall survival | Local control | Late toxicity at reporting |
|---|---|---|---|---|---|---|
| Dionisi et al. (53) | 17 | Protons | 60 (30.6–66) | 54.4% (18 months); 59.3% (radical cases) | LC 66.6% (18 months); 72.9% (radical cases) | 23.5% ≥ G3, 1 carotid blowout (G5) |
| Hu et al. (54) | 75 | Carbon ions | 50–60 | 98.1% (1 yr) | LRFS 86.6% (1 yr)*; RRFS 97.9% (1 yr) | 7 necrosis at tumor bed, including 1 carotid blowout (G5) |
| Lin et al. (19) | 16 | Protons | 62.8 (59.4–70.2) | 50% (2 yr) (83% for optimal target coverage) | DFS 50% (2 yr); LC 50% (2 yr) (LC 83% for optimal target coverage) | 1 osteonecrosis; 1 chronic ulceration (nasopharynx); 1 trismus; 2 chronic serous otitis |
| Feehan et al. (24) | 11 | Helium/neon ions | 50 (31.8–62.3) | 59% (3 yr); 31% (5 yr) | LC 45% (at reporting) (median follow up 28.1 months) | Trismus, hypopituitarism, 1 carotid bleeding (recovered) |
*, there were 21 local failures at the time of analysis despite the 86.6% LRFS at 1 year. DFS, disease free survival; LC, local control; LRFS, local relapse free survival; RRFS, regional relapse free survival.
The future development of proton/heavy-ion therapy in the salvage of NPC local failure
High level evidence gathered through carefully designed clinical trials are often demanded to truly demonstrate the effectiveness and efficacy of proton/heavy-ion therapy (61-63). There are currently five CIRT (and no proton therapy) trials registered in clinicaltrials.gov targeting locally recurrent NPC, all from Shanghai Proton and Heavy Ion Center. One of them looks at the role of two drugs used in combination with CIRT but is not yet recruiting. Another trial studies the prognostic value of FLT PET/CT for recurrent NPC patients after CIRT with no update since 2018 September. The other three trials aim at determining the MTD and its effectiveness in treating recurrent NPC patients who have received IMRT as their primary treatment. Each trial looks at a slightly different (and mostly overlapping) dose ranging from 52.5 to 63 GyE, at 2.5 or 3 GyE per fraction, and all are in combination with cisplatin chemotherapy. Their outcome measures are the number of treatment-related adverse events, OS and PFS. Two of the trials have already been terminated due to slow accrual, and the only active one trying out 54 to 63 GyE (3 GyE per fraction) has stopped recruiting. More clinical trials are definitely welcome for this modality in recurrent NPC treatment.
Given the highly successful treatment outcome of primary NPC with IMRT combined with chemotherapy, as well as the high capital cost and associated uncertainties in proton/heavy-ion dose delivery and radio-biological effectiveness, it is unlikely that proton/heavy-ion therapy will replace IMRT and become the major modality for treating primary NPC in near future. However, in the challenging scenario of recurrent NPC where IMRT cannot triumph, the energy deposition characteristics of proton/heavy-ion make them a very attractive alternative. While proton/heavy-ion beams have the potential to greatly reduce the integral dose and better spare the normal tissue near the target volume, their ability to deliver a highly conformal dose distribution is being hindered by their extreme sensitivity to range uncertainty. As usually only 2 to 3 beams are used, the selection of beam angles and the robustness consideration during optimization could also have a significant impact on the dosimetric quality of the treatment plan. In addition, the uncertainty in RBE plays a role in the planning and the final clinical outcomes. For carbon ions a large variation of RBE (typically from 2 to 5) along their range is observed, and many factors like fraction size, end point, tissue types, etc., are all affecting the actual RBE value. The associated large uncertainty in RBE casts a shallow on the expectation of the actual treatment outcome. In the case of protons, while the variation in RBE appears to be smaller, the use of a single RBE has been raising concerns with the emerging clinical data (22,64). All these issues might have contributed to the lack of significant improvement in treatment outcomes of recurrent NPC with proton/heavy-ion therapy after more than 30 years of usage. It would be difficult to fully exploit the dose sculpting capability of proton/carbon-ion therapy if these uncertainties are not addressed properly. Having said that, the performance of proton/heavy-ion therapy is in general superior to using X-ray IMRT despite these issues and limitation, they should still be the modality of choice for the right patients. The means to reduce the negative impact in range uncertainty should be a major focus of future study. On-going research includes the study of using dual energy CT (65) or even proton radiology (66) to reduce or eliminate CT number calibration uncertainty, as well as the verification of proton range during delivery using prompt gamma imaging (67).
Another area that has raised much interest with proton/heavy-ion therapy is FLASH irradiation. Many studies have shown that when the radiation dose is delivered in an ultra-high dose rate (more than 40 Gy per second) tumor cells can be eradicated with much less normal tissue toxicity even if the same dose is taken. FLASH is not feasible with X-ray radiotherapy on deep-seated tumor in human with the current linear accelerator due to dose rate limitation, and most of the energy would still be wasted in the normal tissue before and behind the target volume (uniform dose using cross fire technique will be out of question unless all the beams from multiple angles can be “fired” at once). However, the high dose rate with a uniform dose distribution in the target region can be achieved with even a single proton/heavy-ion beam and this opens up a completely different area of opportunity in radiotherapy. A simple single SOBP produced using a ridge filter (a range-modulating wheel would take too long to “spread” the Bragg peak out for FLASH) to cover a fairly regular region encompassing the whole target with sufficient margin for range uncertainty is all that is required. There would be little need to spare the very small amount of critical structures abutting the target volume or to worry about the difference in RBE, which is yet unknown but likely to be less an issue in FLASH mode. Although the actual mechanism of FLASH is yet to be determined, the first treatment with FLASH on human had already been conducted with electron beam (68). A FLASH proton therapy delivery system is also in place for small animal testing (69), and the Netherlands Proton Therapy Center has been able to deliver FLASH radiation with their clinical proton therapy system (70). Any success in FLASH irradiation mode will be most beneficial to situation like recurrent NPC where the major obstacle is the tolerance of nearby critical structures limiting the high dose coverage to the whole target volume.
The use of proton/heavy-ion therapy is in constant competition with the advancement in X-ray radiotherapy. In addition to the high capital (and probably running) cost, there are physical and radiobiological issues to be tackled. As we might be approaching the physical limit of X-ray radiotherapy, the interest in proton/heavy-ion therapy has grown dramatically and such system is becoming increasingly available around the world. With all the issues and questions yet to be answered there is substantial room for further improvement. Researches and studies towards their development are being conducted and there is high hope that their true potential can be realized in the near future and bring about another major breakthrough in radiotherapy like IMRT in photon therapy.
Acknowledgments
Funding: None
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Nasopharynx Cancer for the series “Precision Radiotherapy in Nasopharyngeal Carcinoma”. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/anpc-20-7). The series “Precision Radiotherapy in Nasopharyngeal Carcinoma” was commissioned by the editorial office without any funding or sponsorship. WTN served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Annals of Nasopharynx Cancer from Aug 2019 to Aug 2021. The author has no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lee AW, Ng WT, Chan YH, et al. The battle against nasopharyngeal cancer. Radiother Oncol 2012;104:272-8. [Crossref] [PubMed]
- Lee AW, Ng WT, Chan LL, et al. Evolution of treatment for nasopharyngeal cancer--success and setback in the intensity-modulated radiotherapy era. Radiother Oncol 2014;110:377-84. [Crossref] [PubMed]
- Setton J, Han J, Kannarunimit D, et al. Long-term patterns of relapse and survival following definitive intensity-modulated radiotherapy for non-endemic nasopharyngeal carcinoma. Oral Oncol 2016;53:67-73. [Crossref] [PubMed]
- Ng WT, Lee MC, Hung WM, et al. Clinical outcomes and patterns of failure after intensity-modulated radiotherapy for nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 2011;79:420-8. [Crossref] [PubMed]
- Au KH, Ngan RKC, Ng AWY, et al. Treatment outcomes of nasopharyngeal carcinoma in modern era after intensity modulated radiotherapy (IMRT) in Hong Kong: A report of 3328 patients (HKNPCSG 1301 study). Oral Oncol 2018;77:16-21. [Crossref] [PubMed]
- Lee AWM, Ng WT, Chan JYW, et al. Management of locally recurrent nasopharyngeal carcinoma. Cancer Treat Rev 2019;79:101890 [Crossref] [PubMed]
- Ng WT, Wong ECY, Cheung AKW, et al. Patterns of care and treatment outcomes for local recurrence of NPC after definite IMRT-A study by the HKNPCSG. Head Neck 2019;41:3661-9. [Crossref] [PubMed]
- Ng WT, Lee MC, Fung NT, et al. Dose volume effects of re-irradiation for locally recurrent nasopharyngeal carcinoma. Head Neck 2020;42:180-7. [Crossref] [PubMed]
- Wong E, Hung J, Ng WT. Re-irradiation for recurrent NPC: is treatment merited at all? Ann Nasopharynx Cancer 2018;2:9.
- Leong YH, Soon YY, Lee KM, et al. Long-term outcomes after reirradiation in nasopharyngeal carcinoma with intensity-modulated radiotherapy: A meta-analysis. Head Neck 2018;40:622-31. [Crossref] [PubMed]
- Available online: https://www.ptcog.ch/
- ASTRO Proton Beam Therapy Model Policy. Available online: https://www.astro.org/uploadedFiles/_MAIN_SITE/Daily_Practice/Reimbursement/Model_Policies/Content_Pieces/ASTROPBTModelPolicy.pdf (accessed 23 Mar 2020).
- National Comprehensive Cancer Network. NCCN guidelines for treatment of cancer by site – Head and Neck Cancers version 1.2020. Available online: https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf
- Mohamad O, Yamada S, Durante M. Clinical Indications for Carbon Ion Radiotherapy. Clin Oncol (R Coll Radiol) 2018;30:317-29. [Crossref] [PubMed]
- Kamada T, Tsujii H, Blakely EA, et al. Carbon ion radiotherapy in Japan: an assessment of 20 years of clinical experience. Lancet Oncol 2015;16:e93-e100. [Crossref] [PubMed]
- Wilkens JJ, Oelfke U. Direct comparison of biologically optimized spread-out bragg peaks for protons and carbon ions. Int J Radiat Oncol Biol Phys 2008;70:262-6. [Crossref] [PubMed]
- Ohno T. Particle radiotherapy with carbon ion beams. EPMA J 2013;4:9. [Crossref] [PubMed]
- Chu WT: Overview of Light-Ion Beam Therapy. Columbus-Ohio ICRU-IAEA Meeting, 2006.
- Lin R, Slater JD, Yonemoto LT, et al. Nasopharyngeal carcinoma: repeat treatment with conformal proton therapy--dose-volume histogram analysis. Radiology 1999;213:489-94. [Crossref] [PubMed]
- Paganetti H. Relative biological effectiveness (RBE) values for proton beam therapy. Variations as a function of biological endpoint, dose, and linear energy transfer. Phys Med Biol 2014;59:R419-72. [Crossref] [PubMed]
- Chaudhary P, Marshall TI, Perozziello FM, et al. Relative biological effectiveness variation along monoenergetic and modulated Bragg peaks of a 62-MeV therapeutic proton beam: a preclinical assessment. Int J Radiat Oncol Biol Phys 2014;90:27-35. [Crossref] [PubMed]
- Jones B, McMahon SJ, Prise KM. The Radiobiology of Proton Therapy: Challenges and Opportunities Around Relative Biological Effectiveness. Clin Oncol (R Coll Radiol) 2018;30:285-92. [Crossref] [PubMed]
- Barten DL, Tol JP, Dahele M, et al. Comparison of organ-at-risk sparing and plan robustness for spot-scanning proton therapy and volumetric modulated arc photon therapy in head-and-neck cancer. Med Phys 2015;42:6589-98. [Crossref] [PubMed]
- Feehan PE, Castro JR, Phillips TL, et al. Recurrent locally advanced nasopharyngeal carcinoma treated with heavy charged particle irradiation. Int J Radiat Oncol Biol Phys 1992;23:881-4. [Crossref] [PubMed]
- Unkelbach J, Chan TC, Bortfeld T. Accounting for range uncertainties in the optimization of intensity modulated proton therapy. Phys Med Biol 2007;52:2755-73. [Crossref] [PubMed]
- Liu W, Frank SJ, Li X, et al. Effectiveness of robust optimization in intensity-modulated proton therapy planning for head and neck cancers. Med Phys 2013;40:051711 [Crossref] [PubMed]
- Schneider W, Bortfeld T, Schlegel W. Correlation between CT numbers and tissue parameters needed for Monte Carlo simulations of clinical dose distributions. Phys Med Biol 2000;45:459-78. [Crossref] [PubMed]
- Phan J, Sio TT, Nguyen TP, et al. Reirradiation of Head and Neck Cancers With Proton Therapy: Outcomes and Analyses. Int J Radiat Oncol Biol Phys 2016;96:30-41. [Crossref] [PubMed]
- Romesser PB, Cahlon O, Scher ED, et al. Proton Beam Reirradiation for Recurrent Head and Neck Cancer: Multi-institutional Report on Feasibility and Early Outcomes. Int J Radiat Oncol Biol Phys 2016;95:386-95. [Crossref] [PubMed]
- McDonald MW, Zolali-Meybodi O, Lehnert SJ, et al. Reirradiation of Recurrent and Second Primary Head and Neck Cancer With Proton Therapy. Int J Radiat Oncol Biol Phys 2016;96:808-19. [Crossref] [PubMed]
- Yang M, Zhu XR, Park PC, et al. Comprehensive analysis of proton range uncertainties related to patient stopping-power-ratio estimation using the stoichiometric calibration. Phys Med Biol 2012;57:4095-115. [Crossref] [PubMed]
- Pflugfelder D, Wilkens JJ, Oelfke U. Worst case optimization: a method to account for uncertainties in the optimization of intensity modulated proton therapy. Phys Med Biol 2008;53:1689-700. [Crossref] [PubMed]
- Yang Z, Li H, Li Y, et al. Statistical evaluation of worst-case robust optimization intensity-modulated proton therapy plans using an exhaustive sampling approach. Radiat Oncol 2019;14:129. [Crossref] [PubMed]
- Unkelbach J, Bortfeld T, Martin BC, et al. Reducing the sensitivity of IMPT treatment plans to setup errors and range uncertainties via probabilistic treatment planning. Med Phys 2009;36:149-63. [Crossref] [PubMed]
- Fredriksson A, Forsgren A, Hardemark B. Minimax optimization for handling range and setup uncertainties in proton therapy. Med Phys 2011;38:1672-84. [Crossref] [PubMed]
- van der Laan HP, van de Water TA, van Herpt HE, et al. The potential of intensity-modulated proton radiotherapy to reduce swallowing dysfunction in the treatment of head and neck cancer: A planning comparative study. Acta Oncol 2013;52:561-9. [Crossref] [PubMed]
- van de Water TA, Bijl HP, Schilstra C, et al. The potential benefit of radiotherapy with protons in head and neck cancer with respect to normal tissue sparing: a systematic review of literature. Oncologist 2011;16:366-77. [Crossref] [PubMed]
- Kandula S, Zhu X, Garden AS, et al. Spot-scanning beam proton therapy vs intensity-modulated radiation therapy for ipsilateral head and neck malignancies: a treatment planning comparison. Med Dosim 2013;38:390-4. [Crossref] [PubMed]
- Apinorasethkul O, Kirk M, Teo K, et al. Pencil beam scanning proton therapy vs rotational arc radiation therapy: A treatment planning comparison for postoperative oropharyngeal cancer. Med Dosim 2017;42:7-11. [Crossref] [PubMed]
- Liu SW, Li JM, Chang JY, et al. A treatment planning comparison between proton beam therapy and intensity-modulated x-ray therapy for recurrent nasopharyngeal carcinoma. J Xray Sci Technol 2010;18:443-50. [Crossref] [PubMed]
- Wang L, Hu J, Liu X, et al. Intensity-modulated carbon-ion radiation therapy versus intensity-modulated photon-based radiation therapy in locally recurrent nasopharyngeal carcinoma: a dosimetric comparison. Cancer Manag Res 2019;11:7767-77. [Crossref] [PubMed]
- Delaney AR, Dahele M, Tol JP, et al. Using a knowledge-based planning solution to select patients for proton therapy. Radiother Oncol 2017;124:263-70. [Crossref] [PubMed]
- Langendijk JA, Lambin P, De Ruysscher D, et al. Selection of patients for radiotherapy with protons aiming at reduction of side effects: the model-based approach. Radiother Oncol 2013;107:267-73. [Crossref] [PubMed]
- Mahajan A. Patient selection for proton therapy: a clinicians view. Radiother Oncol 2016;119 S1:SP-0009.
- Quik EH, Feenstra TL, Postmus D, et al. Individual patient information to select patients for different radiation techniques. Eur J Cancer 2016;62:18-27. [Crossref] [PubMed]
- Mee T, Kirkby NF, Kirkby KJ. Mathematical Modelling for Patient Selection in Proton Therapy. Clin Oncol (R Coll Radiol) 2018;30:299-306. [Crossref] [PubMed]
- Wang CC. Re-irradiation of recurrent nasopharyngeal carcinoma--treatment techniques and results. Int J Radiat Oncol Biol Phys 1987;13:953-6. [Crossref] [PubMed]
- Lee AW, Law SC, Foo W, et al. Retrospective analysis of patients with nasopharyngeal carcinoma treated during 1976-1985: survival after local recurrence. Int J Radiat Oncol Biol Phys 1993;26:773-82. [Crossref] [PubMed]
- Chan OS, Sze HC, Lee MC, et al. Reirradiation with intensity-modulated radiotherapy for locally recurrent T3 to T4 nasopharyngeal carcinoma. Head Neck 2017;39:533-40. [Crossref] [PubMed]
- Lee AW, Foo W, Law SC, et al. Total biological effect on late reactive tissues following reirradiation for recurrent nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 2000;46:865-72. [Crossref] [PubMed]
- Ang KK, Jiang GL, Feng Y, et al. Extent and kinetics of recovery of occult spinal cord injury. Int J Radiat Oncol Biol Phys 2001;50:1013-20. [Crossref] [PubMed]
- Sulman EP, Schwartz DL, Le TT, et al. IMRT reirradiation of head and neck cancer-disease control and morbidity outcomes. Int J Radiat Oncol Biol Phys 2009;73:399-409. [Crossref] [PubMed]
- Dionisi F, Croci S, Giacomelli I, et al. Clinical results of proton therapy reirradiation for recurrent nasopharyngeal carcinoma. Acta Oncol 2019;58:1238-45. [Crossref] [PubMed]
- Hu J, Bao C, Gao J, et al. Salvage treatment using carbon ion radiation in patients with locoregionally recurrent nasopharyngeal carcinoma: Initial results. Cancer 2018;124:2427-37. [Crossref] [PubMed]
- Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys 1991;21:109-22. [Crossref] [PubMed]
- Blanchard P, Garden AS, Gunn GB, et al. Intensity-modulated proton beam therapy (IMPT) versus intensity-modulated photon therapy (IMRT) for patients with oropharynx cancer - A case matched analysis. Radiother Oncol 2016;120:48-55. [Crossref] [PubMed]
- Holliday EB, Frank SJ. Proton therapy for nasopharyngeal carcinoma. Chin Clin Oncol 2016;5:25. [Crossref] [PubMed]
- Jensen AD, Nikoghosyan A, Ellerbrock M, et al. Re-irradiation with scanned charged particle beams in recurrent tumours of the head and neck: acute toxicity and feasibility. Radiother Oncol 2011;101:383-7. [Crossref] [PubMed]
- Mohan R, Das IJ, Ling CC. Empowering Intensity Modulated Proton Therapy Through Physics and Technology: An Overview. Int J Radiat Oncol Biol Phys 2017;99:304-16. [Crossref] [PubMed]
- Kong L, Lu JJ. Reirradiation of locally recurrent nasopharyngeal cancer: history, advances, and promises for the future. Chin Clin Oncol 2016;5:26. [Crossref] [PubMed]
- Mishra MV, Aggarwal S, Bentzen SM, et al. Establishing Evidence-Based Indications for Proton Therapy: An Overview of Current Clinical Trials. Int J Radiat Oncol Biol Phys 2017;97:228-35. [Crossref] [PubMed]
- Lazar AA, Schulte R, Faddegon B, et al. Clinical trials involving carbon-ion radiation therapy and the path forward. Cancer 2018;124:4467-76. [Crossref] [PubMed]
- Odei BCL, Boothe D, Keole SR, et al. A 20-Year Analysis of Clinical Trials Involving Proton Beam Therapy. Int J Part Ther 2017;3:398-406. [Crossref] [PubMed]
- Woodward WA, Amos RA. Proton Radiation Biology Considerations for Radiation Oncologists. Int J Radiat Oncol Biol Phys 2016;95:59-61. [Crossref] [PubMed]
- Yang M, Virshup G, Clayton J, et al. Theoretical variance analysis of single- and dual-energy computed tomography methods for calculating proton stopping power ratios of biological tissues. Phys Med Biol 2010;55:1343-62. [Crossref] [PubMed]
- Poludniowski G, Allinson NM, Evans PM. Proton radiography and tomography with application to proton therapy. Br J Radiol 2015;88:20150134 [Crossref] [PubMed]
- Hueso-González F, Fiedler F, Golnik C, et al. Compton Camera and Prompt Gamma Ray Timing: Two Methods for In Vivo Range Assessment in Proton Therapy. Front Oncol 2016;6:80. [Crossref] [PubMed]
- Bourhis J, Sozzi WJ, Jorge PG, et al. Treatment of a first patient with FLASH-radiotherapy. Radiother Oncol 2019;139:18-22. [Crossref] [PubMed]
- Patriarca A, Fouillade C, Auger M, et al. Experimental Set-up for FLASH Proton Irradiation of Small Animals Using a Clinical System. Int J Radiat Oncol Biol Phys 2018;102:619-26. [Crossref] [PubMed]
- van Marlen P, Dahele M, Folkerts M, et al. Bringing FLASH to the Clinic: Treatment Planning Considerations for Ultrahigh Dose-Rate Proton Beams. Int J Radiat Oncol Biol Phys 2020;106:621-9. [Crossref] [PubMed]
Cite this article as: Lee MCH, Ng WT. Proton/heavy ion therapy in salvage of locally recurrent nasopharyngeal carcinoma. Ann Nasopharynx Cancer 2020;4:4.


