Knowledge-based planning in nasopharyngeal carcinoma
Introduction
Radiation therapy is a widely adopted and effective treatment in nasopharyngeal carcinoma (NPC).
With the complicated disease nature, intensity-modulated radiation therapy (IMRT) and volumetric modulated arc therapy (VMAT) are the key treatment techniques for maximizing cancer control while minimizing toxicity to normal organs (1-3). Inverse planning techniques which optimize the doses to both the planning target volumes (PTVs) and organ-at-risks (OARs) are required for the planning of IMRT and VMAT (4). Universal planning goals can be used to judge whether the plan can meet the acceptance standards, whether the plan could be further improved is dependent on the patient’s anatomy and thus differs from case-to-case. This relies on the planner’s ability to observe and pinpoint particular areas for further improvements and ultimately guide the optimization to obtain better results (5-7). To explore various possibilities of achieving a better plan, some trial-and errors processes are inevitable. Simply knowing what to try and when to stop could largely reduce the time spent on unnecessary trials. Thus not only the quality of the treatment plan but also the planning time are highly dependent to the knowledge and experience of the planners (8,9).
As early as 1980s, automatic planning systems have been experimented to aid the design of computerized radiation treatment plans (10). Those systems mainly translate the knowledge and experience to rules and algorithms that help to automate the tedious and repetitive manual manipulations in the planning process. With the improvement of computer power and speed, these systems have further advanced and in recent years, some of them were developed as commercially available solutions. One example is AutoPlanningTM within Philips Pinnacle TPS (11). Similar to the steps that a human planner would take in planning, the software optimizes the plan iteratively by creating additional ROI and optimization constraints based on transient dose distribution using a fix set of proprietary rules. These systems are typically efficient in generating clinically acceptable plans, however, they offer limited control on the trade-off between target coverage and OAR sparing. To tackle this limitation, multi-criteria optimization (MCO)—another type of auto-planning algorithm—has emerged. As of year 2020, various MCO planning tools are available commercially including Eclipse (Varian) (12), Raystation (RaySearch Laboratories) (13), Erasmus-iCycle (Erasmus MC, Rotterdam) (14), etc. MCO automatically generates a series of Pareto-optimized plans (plans of which no objective quantity can be improved without impairing at least one another) with a variety of trade-offs and the clinician can choose from the pool the one that best suits the patient. Choosing an optimal trade-off could be challenging and requires good clinical knowledge and experiences. As clinical experiences with IMRT/VMAT accumulates over the past two decades, a new data-driven method, known as knowledge-based planning (KBP), has been developed to extract the best clinical judgement and knowledge from prior good cases and apply them to generate new plans automatically (15-24). In the big data era, such approach allows the sharing of knowledge between different oncology centers and shows great promise in the development of fully automated planning with improving planning quality and efficiency.
General overview of KBP
KBP engines are generally comprised of three components: (I) an input library/database consisting of ensembles of prior clinical data, (II) extraction of knowledge from the database and conversion into optimization parameters, (III) an optimization algorithm that uses the optimization parameters determined to guide the creation of a deliverable plan.
Input library/database
The input data may include the planning CT images, delineated structures, planning parameters, dose volumes, patient demographic characteristics, etc. Usually the inputs are restricted to only a particular anatomical site, sometimes even disease type and protocol. These variations could also affect the minimum amount of input plans required for a good KBP system. One would normally expect a larger number of plans required for KBP in head and neck cancers due to the sophisticated and diversification natures of the diseases (many PTVs/OARs, various dose levels, large variation in PTV shapes and locations).
Extraction of knowledge from database
There are two main ways of extracting key knowledge from past data—atlas-based and model-based.
In atlas-based KBP, the reference patient(s) in the database best matching the subject patient to be treated is first identified from whose plans the knowledge is extracted. Various methods have been explored to pick out the best matched patient. A popular approach is to look for maximal similarities between patients in terms of the relative spatial locations of targets and OARs [e.g., overlapping area of the overlap volume histograms (OVH) (25)] which are most critical factors affecting the attainable target coverage and normal tissue sparing. Among the reference patients that are sufficiently similar to the subject patient, one might also want to choose the one with the best plan quality, for instance, choose a plan with minimum OAR dose, but with sufficient target coverage (26).
Model-based KBP, on the other hand, instead of extracting knowledge from only the best matched reference patient(s), incorporates the essence of knowledge and experience of all reference plans in the database into a single prediction model. The model fits upon features and patterns available in the reference plans and output the estimation of the best geometrical configuration for planning or the best dosimetric outcome that should be achievable in the new plan. A large variety of prediction model exist, majority of them are based on Machine learning methods such as regression, random forest, support vector machine, etc. Deep learning, a particular type of machine learning based on artificial neural network, has also been applied in KBP (27) and is starting to gain increasing popularity and has shown significant promise as the next generation of auto-planning algorithm.
Generating optimization parameters
The knowledge extracted in the atlas-based KBP or predictions from model-based KBP are used to generate optimization parameters for optimization of new plans. For instance, the fluence map of the new plan can be obtained by deformation registration of the fluence map of the best matched patient plans in an atlas-based KBP system, dose-volume histogram (DVH) prediction from a model-based KBP can be fed to inverse optimization algorithm to generate new plan. Other examples of knowledge transferred/optimization parameters include beam parameters (e.g., gantry/collimator/couch angles, jaw settings), voxel dose distribution, objective function weights, etc. DVH estimates are by far the most commonly used. However, one potential pitfall in the DVH approach is that DVH contains no geometrical information, as such the plan produced might fulfil all the DVH criteria but still presented with an inferior 3D dose distribution (e.g., slow fall-off). This provokes new research and developments in voxel-based planning. For instance, Chen et al. has recently implemented a convolutional neural network, ResNet, a specialized architecture for imaging and vision purposes, for 3D dose distribution prediction for simultaneous integrated boost (SIB) radiotherapy in NPC (28).
Creation of deliverable plans
The prediction or knowledge transferred can only be used as a guide for further optimization since (I) every patient is inevitably different, no matter how similar with one another; (II) the plan needs to be deliverable, i.e., ones that are physically possible with the modality of interest, e.g., linac, Cyberknife, etc.). Examples of optimization engines include DVH guided inverse-planning algorithms (the most commonly used), voxel-based dose mimicking algorithms [McIntosh et al. (29)/Raystation], etc.
Example of KBP (RapidPlan) applied to NPC IMRT planning
To demonstrate how actually KBP is implemented, we will look at a specific example of using RapidPlanTM (RP) in NPC IMRT planning. This is the custom NPC model that was employed in the study of Chang et al. (30). It had been commissioned and used clinically in our oncology center to assist NPC planning since 2016.
RP in a nutshell
RP is a knowledge-based optimization application that is provided as an integrated option in the Eclipse treatment planning system (Varian Medical Systems, Palo Alto, USA) since release 13.5.
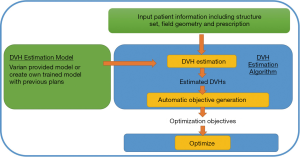
The RP model is first trained using prior high quality plans to establish the relationship between geometry (both anatomy and field arrangement) and dosimetry. The model can be used to estimate the OARs’ DVH in a new plan when given the patient information (structure sets, prescription, field geometry). The DVH estimation (Figure 1) and the automatically generated priorities are then fed into inverse optimizer to generate a deliverable plan (Figure 2).

RP DVH Estimation algorithm (31)
Volume sub-division
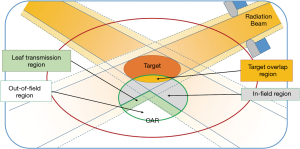
Each OAR is sub-divided into four regions (Figure 3): out of field, in-field, leaf transmission, overlapping region. DVH of the in-field region could be highly variable depending on how the plan is optimized, and will be modelled with the help of prior data. Variations of DVH in other regions are limited and are estimated using relatively crude models.
Evaluation of geometry
The geometry is represented through the construction of the geometry-based expected DVH (GBDVH). Details of GBDVH construction is beyond the scope of this article, more detail can be found in reference (31).
Principle component analysis (PCA)
PCA is performed to both the GBDVH and the in-field DVH to extract principle components (PCs) that maximizes the variance of the DVHs in the training set. PC score (PCS)/coefficient of each plan for each OAR is calculated.
Regression
For each OAR, stepwise-regression is performed to correlate the PCS of GBDVH and PCS of in-field DVH across all plans in the training set.
DVH estimation of new plan
PCS of GBDVH for the new plan is calculated. Using coefficients obtained in the regression, PCS of the in-field DVH can be determined which are in turn used to generate the in-field DVH estimates. By combining DVH estimates from the other OAR sub-volume, DVHs of the OARs can be estimated. Line objectives for OARs are then created along the lower DVH estimation boundaries for inverse optimization along with other fixed objectives manually chosen for PTV (and OAR) structures. The priority of the line objective can also be automatically generated.
Input library for the NPC RP model
In order for the model to be applicable in most clinical cases, the input library should contain enough samples that can represent the majority of the NPC population. In our model, plans of 79 NPC patients, with no differentiation made regarding the stage of disease, were included for training. These NPC patients were given SIB treatments in 35 fractions to three prescription dose levels (70, 63, 56 Gy) using IMRT technique (six MV photon). VMAT was not included in the model because our Institute only used IMRT for NPC treatment and no previous knowledge of using VMAT was available for the KBP model.
PTVs of different dose levels were individually cropped, and were made to separate from each other by 3 mm margins. OARs of left and right side were grouped together (for instance, left and right parotid as “parotid” in the model). Altogether, nine OAR structures that were included in the model. They were Brainstem + 1 mm, Cochlea, Cord + 3 mm, Eye, Lens_PRV, Op_Chiasm + 1 mm, Optic Nerve, Parotid and Temporal Lobe.
Training and refinement of RP model
The DVH Estimation model of each OAR is trained using the plans imported in the library. The statistics of the trained model are summarized in the geometry plot (containing statistics of OAR/target volume, percentage overlapping of OAR to target, PCSs of the GBDVH), regression/residual plots as well as in-field DVH/overall DVH. By analyzing these statistics together with the training log (Figure 4), possible geometric/dosimetric outliers that can impact the reliability of the model can be detected [more information can be found in the manual (31,32)]. Metrics such as regression coefficient of determination, studentized residuals and Cook’s distance in the training log help to pinpoint particular outlier plans that should be removed from the model. To further improve the reliability and precision of DVH estimation, the model was re-trained iteratively and recursively (33), i.e., the model was used to re-plan cases if the estimated DVHs of OARs outperform that of the input plans. The input plans were then replaced by these re-optimized plans and re-training was performed. Finally, all cases were re-planned with the re-trained model and all the new data were imported to train and create a new RP model. As illustrated by the example shown in Figure 4A, a strong correlation is found between the geometry and dosimetric PCS, indicating that the model is well-configured. The model performance could probably be further enhanced by adding more quality plans into the input library.

Performance and validation of the NPC RP model
The performance of the RP model was evaluated against manual planning for a set of twenty NPC patients that are independent of the training set (30). In general, the target coverage for both the manual and RP plans (plans generated using line constraints and priorities suggested by the RP model) were similar. The mean doses of OARs were generally reduced with the help of DVH line constraints. However, control on the maximum OAR dose was inferior to the manual plans with about half of the plans not fulfilling the acceptance criteria of the top priority serial OAR structures [the summary of the target and normal tissue constraints are described as in the article (30)]. Nevertheless, the difference was very small and all these plans could be made acceptable with minimal manual adjustments. The planning time using RP plan followed by manual adjustments is still significantly less than the time required for full manual plans: 64 vs. 295 minutes. These results demonstrated the current status and feasibility of RP employed in IMRT planning of NPC. RP does not limit the application of VMAT planning even though the model is configured using IMRT plans only, however, the result concerning the IMRT KBP model might not be applicable to VMAT plans and the reader should interpret these results with cautions.
Use of KBP in head and neck cancers
Previous reports on KBP are based on two main types of validation studies comparing KBP-predicted dose metrics, or KBP-produced dose distribution with those of manual clinical plans. General findings suggest that KBP methods are capable of achieving clinically acceptable target volume coverage with improvements in OAR sparing. Universally, planning time was found to be significantly reduced, especially for complex cases. In head and neck cases, various studies have shown sparing to parotids, submandibular glands, oral cavity, and swallowing muscles (such as pharyngeal constrictor muscles); a study by Tol et al. (21) showed that significant improvement in mean parotid dose (more than 4 Gy) can be achieved with RP when compared with the original plans from an older series, reflecting most benefit for inexperienced centers. It is also noted that plan quality is less consistent for some “outlier” cases. In building the model, anatomical features of PTV and OAR and their spatial relationships are important parameters, such as median OAR and PTV distance, proportion of OAR volume within a specific distance range or overlapping with PTV etc. (34-36). It is controversial as to the number of cases required to train a model as it is expected that head and neck cancers contain higher complexity and likely require more training cases, compared with other tumor types such as prostate cancer with fewer OAR’s to consider.
Automated treatment planning has also been adopted in the context of clinical trials. The Radiation Therapy Oncology Group (RTOG) 0920 and 3501 utilized a model which incorporated data from head and neck cancer patients who had previously received helical tomotherapy or VMAT, to re-plan VMAT patients who were recruited into these clinical trials. Model plans were shown to improve OAR sparing compared with manual plans with maintenance of clinically acceptable dose uniformity. A clear advantage is significantly improved planning efficiency (37-39).
Other applications on KBP
Patient selection for different modality
The predictive power of KBP in dosimetric outcome can also be exploited for patient selection purposes. Due to the limited availability and high cost of proton therapy, as well as the capability of modern IMRT techniques, there is always a need to select the right patients who can benefit most from proton therapy. Usually treatment plans of these two modalities need to be produced, optimized and compared, which is time and labor intensive. Delaney et al. (40) demonstrated the idea of patient selection by KBP-predicted mean OAR doses for 10 head and neck cancer patients. The mean doses of parotid glands, contralateral submandibular gland and swallowing muscles were predicted for both intensity-modulated proton therapy (IMPT) and IMRT plans and then compared. Using 6 Gy as the threshold mean dose difference for selection, the method identified four out of five eligible patients (out of a total of ten) for IMPT, and four out of five that would not qualify. The study was subject to the limited capability of applying the KBP model on proton therapy as the KBP algorithm was not yet designed to handle proton beam characteristics, it did however clearly demonstrate the potential of KBP in patient selection to receive the most appropriate treatment modality.
Quality control of IMRT planning
A study by Zhou et al. utilizes knowledge library of reference plans for the quality control of IMRT planning (25). New measures derived from OVH and DVH were used as additional parameters to control IMRT plan quality. Twenty-eight NPC IMRT plans were included and compared against one another according to these new measures; and those for which a better “reference” plan could be found were re-optimized using reference plan DVHs as additional objectives. Significant improvement could be achieved for these plans; in particular, the parotid median dose was reduced by 3.4 Gy on average. The method successfully identified the sub-optimal plans and provided an easy mean for improvement.
IMRT plan quality check is also an important issue in a multi-center clinical trial setting. As sub-optimal plans that still achieve the minimum plan acceptance criteria are usually not considered in trial’s stratification, they can introduce bias and affect final trial outcomes. To demonstrate the use of KBP for planning quality assurance (QA), Tol et al. (41) produced KBP plans for 100 head and neck cases submitted by thirteen different institutes participating in the EORTC-1219-DAHANCA-29 trial, and compared the difference in the mean OAR doses. Only the dose to parotid/submandibular glands and swallowing muscles were compared as the serial OARs constraints must be met for plan submission. They found that the mean dose could be improved by more than 3 Gy for 293/570 OARs. In fact, the mean OAR doses predicted by the knowledge-based model are found to be sufficiently close to that can be achieved in the optimized KBP-plan so that the predicted doses can be used directly for QA purposes without needing to produce a KBP plan at all. Using DVH prediction alone identified 60 submitted plans that the OAR mean dose can be potentially reduced by more than 3 Gy. The KBP prediction provides a quick means for plan QA and the predicted DVH can also be passed to the institutes for plan re-optimization if needed.
Evaluation of dosimetric consequences due to contouring inconsistencies
A study utilized automatically generated treatment plans to evaluate the impact on contour variation was reported by Lim et al. (42). Twenty-two residents contoured the clinical target volumes of different dose levels (54.12, 59.4 and 69.96 Gy), both parotids and cochleae for a T1N1 NPC patient and the contours were compared with the “gold standard” contours created by two expert oncologists. Sixty-seven VMAT plans with four full coplanar arcs were generated with KBP model for four different combinations of resident-drawn and gold-standard PTV and OAR contours. Analysis of the dosimetric indices (PTV D98 and OAR Dmean) provided clinically meaningful conclusion on the consequence of contouring discrepancy, and the inadequacy of the commonly-used geometric indices for contour evaluation (poor correlation between geometric and dosimetric indices with R2 <0.2 for 61% of the correlations studied). The authors of this study recommended using KBP-produced plans for future contour evaluation studies.
Limitations and future developments
As suggested by the name, KBP approach draws from the experience of previous high quality plans. The KBP performance will suffer if poor quality plans have been used to construct the model library. Usually individual institutions construct their own library based on their own treatment protocols with specific prescription dose levels, fractionations, OAR constraints, contouring convention, etc. The KBP plans thus produced would be limited by the institute’s previous planning capability, and the models may not be transferrable to another institute using different protocols. Panettieri et al. (43) demonstrated the feasibility of constructing a universal KBP model for prostate cancer trained with 110 treatment plans contributed from five centers employing different treatment protocols, and then distributed and revalidated by eight centers. A standardized OAR constraint was developed in the process and the KBP model was able to produce quality plans with general improvement in OAR sparing despite the variations in target dose prescription and fractionation scheme. We believe future KBP algorithm will be developed to accommodate a large diversity of treatment schemes, dose constraints, modalities, and even with tuning features; so that with sufficient number and variety of training plans, the resultant model will be sufficiently flexible and easily transferrable to a large number of users with different planning aims.
Conclusions
KBP could improve the efficiency of IMRT planning for NPC patients and produce less planner dependent treatment plan with good quality, although some manual touch-up was occasionally needed for the KBP plans to meet the clinical acceptance criteria. The performance of the model could be further improved by adding more quality data and tuning of the model, potentially removing the need of manual touch-up. With the help of auto-contouring (44) and scripting, fully automated planning might become plausible. Such potentials would be worthy for further exploration. The benefit of improved planning quality and efficiency can also be enjoyed by a large number of patients with the construction of universal KBP models that are highly flexible and transferable to different radiotherapy centers with limited resources.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Nasopharynx Cancer for the series “Precision Radiotherapy in Nasopharyngeal Carcinoma”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/anpc-20-12). The series “Precision Radiotherapy in Nasopharyngeal Carcinoma” was commissioned by the editorial office without any funding or sponsorship. WTN served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Annals of Nasopharynx Cancer from August 2019 to August 2021. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kam MK, Chau RM, Suen J, et al. Intensity-modulated radiotherapy in nasopharyngeal carcinoma: dosimetric advantage over conventional plans and feasibility of dose escalation. Int J Radiat Oncol Biol Phys 2003;56:145-57. [Crossref] [PubMed]
- Ng WT, Lee MC, Hung WM, et al. Clinical outcomes and patterns of failure after intensity-modulated radiotherapy for nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 2011;79:420-8. [Crossref] [PubMed]
- Sun Y, Guo R, Yin WJ, et al. Which T category of nasopharyngeal carcinoma may benefit most from volumetric modulated arc therapy compared with step and shoot intensity modulated radiation therapy. PLoS One 2013;8:e75304 [Crossref] [PubMed]
- Webb S. The physical basis of IMRT and inverse planning. Br J Radiol 2003;76:678-89. [Crossref] [PubMed]
- Zhang Q, Peng Y, Song X, et al. Dosimetric evaluation of automatic and manual plans for early nasopharyngeal carcinoma to radiotherapy. Med Dosim 2020;45:e13-20. [Crossref] [PubMed]
- Carls JL, Mendenhall WM, Morris CG, et al. External auditory canal stenosis after radiation therapy. Laryngoscope 2002;112:1975-8. [Crossref] [PubMed]
- Krayenbuehl J, Zamburlini M, Ghandour S, et al. Planning comparison of five automated treatment planning solutions for locally advanced head and neck cancer. Radiat Oncol 2018;13:170. [Crossref] [PubMed]
- Batumalai V, Jameson MG, Forstner DF, et al. How important is dosimetrist experience for intensity modulated radiation therapy? A comparative analysis of a head and neck case. Pract Radiat Oncol 2013;3:e99-106. [Crossref] [PubMed]
- Nelms BE, Robinson G, Markham J, et al. Variation in external beam treatment plan quality: an inter-institutional study of planners and planning systems. Pract Radiat Oncol 2012;2:296-305. [Crossref] [PubMed]
- Ge Y, Wu QJ. Knowledge-based planning for intensity-modulated radiation therapy: a review of data-driven approaches. Med Phys 2019;46:2760-75. [Crossref] [PubMed]
- Hazell I, Bzdusek K, Kumar P, et al. Automatic planning of head and neck treatment plans. J Appl Clin Med Phys 2016;17:272-82. [Crossref] [PubMed]
- Miguel-Chumacero E, Currie G, Johnston A, et al. Effectiveness of Multi-Criteria Optimization-based Trade-Off exploration in combination with RapidPlan for head & neck radiotherapy planning. Radiat Oncol 2018;13:229. [Crossref] [PubMed]
- Craft DL, Hong TS, Shih HA, et al. Improved planning time and plan quality through multicriteria optimization for intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys 2012;82:e83-90. [Crossref] [PubMed]
- Voet PW, Dirkx ML, Breedveld S, et al. Toward fully automated multicriterial plan generation: a prospective clinical study. Int J Radiat Oncol Biol Phys 2013;85:866-72. [Crossref] [PubMed]
- Wu B, McNutt T, Zahurak M, et al. Fully automated simultaneous integrated boosted-intensity modulated radiation therapy treatment planning is feasible for head-and-neck cancer: a prospective clinical study. Int J Radiat Oncol Biol Phys 2012;84:e647-53. [Crossref] [PubMed]
- Zhu X, Ge Y, Li T, et al. A planning quality evaluation tool for prostate adaptive IMRT based on machine learning. Med Phys 2011;38:719-26. [Crossref] [PubMed]
- Moore KL, Brame RS, Low DA, et al. Experience-based quality control of clinical intensity-modulated radiotherapy planning. Int J Radiat Oncol Biol Phys 2011;81:545-51. [Crossref] [PubMed]
- Appenzoller LM, Michalski JM, Thorstad WL, et al. Predicting dose-volume histograms for organs-at-risk in IMRT planning. Med Phys 2012;39:7446-61. [Crossref] [PubMed]
- Chanyavanich V, Das SK, Lee WR, et al. Knowledge-based IMRT treatment planning for prostate cancer. Med Phys 2011;38:2515-22. [Crossref] [PubMed]
- Good D, Lo J, Lee WR, et al. A knowledge-based approach to improving and homogenizing intensity modulated radiation therapy planning quality among treatment centers: an example application to prostate cancer planning. Int J Radiat Oncol Biol Phys 2013;87:176-81. [Crossref] [PubMed]
- Tol JP, Delaney AR, Dahele M, et al. Evaluation of a knowledge-based planning solution for head and neck cancer. Int J Radiat Oncol Biol Phys 2015;91:612-20. [Crossref] [PubMed]
- Delaney AR, Tol JP, Dahele M, et al. Effect of dosimetric outliers on the performance of a commercial knowledge-based planning solution. Int J Radiat Oncol Biol Phys 2016;94:469-77. [Crossref] [PubMed]
- Fogliata A, Belosi F, Clivio A, et al. On the pre-clinical validation of a commercial model-based optimisation engine: application to volumetric modulated arc therapy for patients with lung or prostate cancer. Radiother Oncol 2014;113:385-91. [Crossref] [PubMed]
- Masi K, Archer P, Jackson W, et al. Knowledge-based treatment planning and its potential role in the transition between treatment planning systems. Med Dosim 2018;43:251-7. [Crossref] [PubMed]
- Zhou Z, Chen Y, Yu Z, et al. A study of quality control method for IMRT planning based on prior knowledge and novel measures derived from both OVHs and DVHs. Biomed Mater Eng 2014;24:3479-85. [Crossref] [PubMed]
- Wu B, Pang D, Simari P, et al. Using overlap volume histogram and IMRT plan data to guide and automate VMAT planning: a head-and-neck case study. Med Phys 2013;40:021714 [Crossref] [PubMed]
- Nguyen D, Long T, Jia X, et al. A feasibility study for predicting optimal radiation therapy dose distributions of prostate cancer patients from patient anatomy using deep learning. Sci Rep 2019;9:1076. [Crossref] [PubMed]
- Chen X, Men K, Li Y, et al. A feasibility study on an automated method to generate patient-specific dose distributions for radiotherapy using deep learning. Med Phys 2019;46:56-64. [Crossref] [PubMed]
- McIntosh C, Welch M, McNiven A, et al. Fully automated treatment planning for head and neck radiotherapy using a voxel-based dose prediction and dose mimicking method. Phys Med Biol 2017;62:5926-44. [Crossref] [PubMed]
- Chang AT, Hung AW, Cheung FW, et al. Comparison of planning quality and efficiency between conventional and knowledge-based algorithms in nasopharyngeal cancer patients using intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys 2016;95:981-90. [Crossref] [PubMed]
- DVH Estimation Algorithm for RapidPlan. In: Eclipse 16.0 Photon and Electron Algorithms Reference Guide. Palo Alto: Varian Medical Systems, 2019.
- Configuring DVH Estimation models for RapidPlan. In: Eclipse 16.0 Photon and Electron Reference Guide. Palo Alto: Varian Medical Systems, 2019.
- Fogliata A, Cozzi L, Reggiori G, et al. RapidPlan knowledge based planning: iterative learning process and model ability to steer planning strategies. Radiat Oncol 2019;14:187. [Crossref] [PubMed]
- Yuan L, Ge Y, Lee WR, et al. Quantitative analysis of the factors which affect the interpatient organ-at-risk dose sparing variation in IMRT plans. Med Phys 2012;39:6868-78. [Crossref] [PubMed]
- Yuan L, Wu QJ, Yin FF, et al. Incorporating single-side sparing in models for predicting parotid dose sparing in head and neck IMRT. Med Phys 2014;41:021728 [Crossref] [PubMed]
- Zhang J, Wu QJ, Xie T, et al. An ensemble approach to knowledge-based intensity-modulated radiation therapy planning. Front Oncol 2018;8:57. [Crossref] [PubMed]
- James JA, Melancon D, Carter R, et al. Evaluation of a knowledge based planning model for head and neck cancer patients treated in the setting of a clinical trial. Int J Radiat Oncol Biol Phys 2016;94:889-90. [Crossref]
- Younge KC, Marsh RB, Owen D, et al. Improving quality and consistency in NRG oncology radiation therapy oncology group 0631 for spine radiosurgery via knowledge-based planning. Int J Radiat Oncol Biol Phys 2018;100:1067-74. [Crossref] [PubMed]
- Li N, Carmona R, Sirak I, et al. Highly efficient training, refinement, and validation of a knowledge-based planning quality-control system for radiation therapy clinical trials. Int J Radiat Oncol Biol Phys 2017;97:164-72. [Crossref] [PubMed]
- Delaney AR, Dahele M, Tol JP, et al. Using a knowledge-based planning solution to select patients for proton therapy. Radiother Oncol 2017;124:263-70. [Crossref] [PubMed]
- Tol JP, Dahele M, Gregoire V, et al. Analysis of EORTC-1219-DAHANCA-29 trial plans demonstrates the potential of knowledge-based planning to provide patient-specific treatment plan quality assurance. Radiother Oncol 2019;130:75-81. [Crossref] [PubMed]
- Lim TY, Gillespie E, Murphy J, et al. Clinically oriented contour evaluation using dosimetric indices generated from automated knowledge-based planning. Int J Radiat Oncol Biol Phys 2019;103:1251-60. [Crossref] [PubMed]
- Panettieri V, Ball D, Chapman A, et al. Development of a multicentre automated model to reduce planning variability in radiotherapy of prostate cancer. Physics and Imaging in Radiation Oncology 2019;11:34-40. [Crossref]
- Fung NTC, Hung WM, Sze CK, et al. Automatic segmentation for adaptive planning in nasopharyngeal carcinoma IMRT: time, geometrical, and dosimetric analysis. Med Dosim 2020;45:60-5. [Crossref] [PubMed]
Cite this article as: Hung WM, Fung NTC, Chang ATY, Lee MCH, Ng WT. Knowledge-based planning in nasopharyngeal carcinoma. Ann Nasopharynx Cancer 2020;4:6.