
Narrative review: temporal lobe necrosis after radiotherapy for nasopharyngeal carcinoma
Introduction
Nasopharyngeal carcinoma (NPC) has very unbalanced geographic distribution, which is particularly endemic within east and southeast Asia (1,2). NPC is highly sensitive to ionizing radiation, radiotherapy is the mainstay treatment for non-metastatic NPC. Currently, photon-based radiotherapy is the most commonly used, it has progressed from two-dimensional (2D) radiotherapy to 3D-conformal radiotherapy and then to intensity-modulated radiotherapy (IMRT). Locoregional control and overall survival have been improving along with significant reduction of radiation-induced toxicities. It is reported that proton or carbon ion radiotherapy for treating NPC could improve the therapeutic ratio even further. However, proton or carbon ion radiotherapy is pricey and its advantage needs further validation in large scale prospective study, thus its application is not yet widespread. Despite therapeutic toxicity has been greatly reduced when compared with two decades ago due to advances in radiation technique, brain necrosis secondary to radiation is still one of the late complications observed in NPC patients, including brainstem, cerebellum and temporal lobe necrosis (TLN). TLN is the most common type, because the tumor is anatomically adjacent skull base, sometimes infiltrates it, the brain parenchyma neighboring is often exposed in high dose radiation range. The reported incidence of TLN varies widely from 1.9% to 35% (3-10). This variability is related to a number of factors. Firstly, radiotherapy techniques and fraction scheme are heterogeneous among studies. Secondly, the median latency of neurological complication is 4 years or more, the incidence of TLN increases with prolonged follow-up (11,12). Thus, the exact incidence of TLN is difficult to achieve due to different follow-up across studies. Moreover, the increase in the use of imaging examination and improvements in technique are reasons that higher incidence is reported in recent studies. Five-year overall survival of NPC patients is about 80% (13). TLN is severely impair patients’ quality of life. With prolonged survival achieved in NPC patients, TLN has become a more significant clinical problem. This study focuses on TLN secondary to radiation in NPC patients, we reviewed TLN related advances in pathophysiology, risk factors, clinical features and management basing on current literatures. We present the following article in accordance with the Narrative Review reporting checklist (available at http://dx.doi.org/10.21037/anpc-2019-LCMNC-03).
Methods
We did an extensive search of MEDLINE and PubMed for English-language articles published between Jan 1, 1990, and Dec 31, 2019. The search terms included: ‘nasopharyngeal carcinoma’, ‘nasopharynx cancer’, ‘temporal lobe radiation necrosis’, ‘temporal lobe necrosis’, ‘temporal lobe injury’, ‘cerebral necrosis’, ‘brain necrosis’, ‘radiotherapy’, ‘chemotherapy’, ‘pathophysiology’, ‘diagnosis’, ‘treatment’, “clinical trials”, and “meta-analysis”. Current clinical trials or studies were prioritized for selection. After evaluating the relevance of the references that had been selected, main articles were comprised of studies that had been published in the last 10 years. Meanwhile, we did not exclude older, original studies that have been referenced widely and are greatly respected.
Discussion
Pathophysiology and risk factors
Pathophysiology
The pathogenesis of radiation-induced TLN, a type of radiation-induced brain necrosis, is under exploring and still not fully understood. It is predominantly seen in white matter. Currently, the mechanism widely accepted are vascular injury, gliocytes and neuron damage and inflammatory reaction. It is believed that above three processes work together to mediate the changes in radiation-induced brain necrosis.
Ionizing radiation provoke the production of reactive oxygen species (ROS). ROS initial DNA and non-DNA damage, letting cells enter into the process of repair and/or apoptosis (14). This process results in endothelial damage, basement membrane swelling, telangiectasis, and platelet and fibrin thrombi formation which present as increases in capillaries permeability, blood-brain barrier (BBB) disruption and vasogenic edema (15). Afterward, the damaged brain tissue is lack of oxygen, resulting in up-regulation of hypoxia-inducible factor-1α (HIF-1α). HIF-1α is a transactivator of vascular endothelial growth factor (VEGF). It causes augmentative production of VEGF by astrocytes, which results in neo-angiogenesis (16). Most new vessels are immature and dysfunctional, which further increase permeability of capillaries and BBB. Ultimately, all these changes lead to brain edema and necrosis. In human specimens, a previous study found that HIF-1α and VEGF were expressed predominantly in perinecrotic area (17).
In addition to endothelial injury, radiation can directly cause damage of astrocytes, oligodendrocytes, oligodendrocyte progenitors and neural progenitors. On one hand, the number of mature oligodendrocytes and its progenitor cells decreases, which eventually leads to myeline loss and demyelination (18). On the other hands, although neurons are relatively resistant to radiation, recently studies found that apoptosis of neural progenitor and impairment in hippocampal neurogenesis happened after radiation (18,19). This change produces symptoms of memory loss, cognitive impairment in clinical and brain atrophy in radiological. The mechanism is undergoing explored.
Inflammation also plays an important role in TLN. The injury of vascular endothelial, gliocytes and neural genesis result in accumulation of inflammatory cells. Amounts of inflammatory cytokines release, like tumor necrosis factor-α (TNF-α), interleukin-1, interleukin-6 and so on, which further aggravate ischemia, edema, fibrinoid necrosis and white matter necrosis (17).
Risk factors
The incidence of TLN is associated with treatment-related factors, tumor-related factors and individual factors.
Treatment-related factors includes radiation dose, involved volume of temporal lobe, fraction size, radiation technique, chemotherapy, target therapy. Radiation dose is recognized one of the most critical factors affecting TLN. Su et al. reported (20) that TLN is associated with maximal dose (Dmax) and the dose delivered to 1 cubic centimeter volume (D1cc). When Dmax ≥64 Gy or D1cc ≥52 Gy, the incidence of TLN increase with augment of 2.6% and 2.5% per Gy respectively. Radiation dose should be strictly limited in temporal lobe, particularly in nasopharyngeal re-irradiation patients. In terms of involved volume of temporal lobe, a study demonstrated that 5-year incidence of TLN for temporal lobes rV40 <10% or aV40 <5 cc is less than 5%. For those with rV40 ≥15% or aV40 ≥10 cc, incidence of TLN is increased significantly and it is more than 20% (rVX: volume percentage of temporal lobes receiving ≥ X Gy, aVX: absolute volume of temporal lobes receiving ≥ X Gy) (3). A later study found similar trend that patients with aV20 >42.22 cc had significantly higher risk of TLN (21). Fraction size is another radiation factor for TLN. In a study of 1,008 NPC patients, 50.4 Gy in 4.2 Gy per fraction was performed in 621 patients and 60 Gy in 2.5 Gy per fraction was used in 320 patients, the incidence of TLN 18.6% and 4.6% was observed in each group (9). Thus, it’s better to use conventional fraction, which is widely in practice around the world nowadays. Last century, two-dimensional radiotherapy (2D RT) was used via laterally opposed fields in the treatment for NPC. In the 1990s, IMRT emerged. It not only improves survival of NPC patients, but greatly reduces the morbidity of TLN after radiation. A prospective study reported that IMRT reduced the incidence of TLN by 8% (4). Actually, this improvement is result from strict restriction for maximal dose and involved volume of temporal lobes in IMRT when compared with 2D RT. Moreover, particle radiation therapy with special properties of radiophysics could improve dose distribution and conformality of radiation plan. In a dosimetric comparison study of carbon-ion and photon-based IMRT, a significant reduce mean dose was observed in normal tissue including temporal lobe (22). Theoretically, it has the potential of protecting temporal lobe, however, it needs to be proved by clinical study in the future. When it comes to chemotherapy, Lee et al. demonstrated that the 5-year incidence of TLN slightly increase (0% vs 1.3%, no statistical significance) by adding concurrent chemotherapy and no negative impact was found by adding neoadjuvant and adjuvant chemotherapy (23). Recently study by Wang and his colleagues presented consistent results in their predictive model for TLN, no significant negative impact was found by adding chemotherapy (24). Cetuximab is an epidermal growth factor receptor (EGFR) inhibitor. In a phase 2 trial, 7 of 33 NPC patients underwent cetuximab plus IMRT developed into TLN in 3 years (25). The incidence is much higher when compared with historical control, suggesting that TLN occurrence might be exacerbated by concurrent cetuximab. As TLN is a late complication that is irreversible, it should be caution when choose this treatment modality and more study in vivo or retrospectively need to be down to further prove this phenomenon. Lastly, the role of molecular target therapy and immunotherapy in TLN is unclear as related report in literatures was limited.
Tumor stage is one of important tumor-related factors, which reflects the distance between temporal lobe and primary tumor. In a retrospective study, which a median follow-up of 40 months, no patients in T1–2 developed into TLN, while 3.1% in T3 and 13.4% in T4 was observed of TLN (20), suggesting T stage is a risk factor for TLN.
Comorbid factors like hypertension, diabetes, lipidemia, obesity and smoking, which might be contributory factors in the development of TLN are not fully studied in literatures. Furthermore, individual factor like heterogeneity of radio-sensitivity is worthy of notice. Radio-sensitivity not only impacts treatment response of tumor, but might play a role in the incidence of radiation toxicity. A genome-wide association study conducted by Wang et al. found that gene CEP128 might be a genetic susceptibility involved in temporal lobe injury development (26). It provides the novel insight into the underlying mechanisms of radiation-induced brain injury. Studies in vitro and vivo warrants to be done in further exploring in this field.
Clinical features
Clinical presentation
TLN includes asymptomatic and symptomatic cases. Asymptomatic cases which were incidentally diagnosed during follow-up have been reported in 18–45% patients (11,27). For patients with symptoms, the presentations are variable. According to a retrospective study (11), 42.6% patients presented with vague symptoms including mild memory impairment, personality change and/or occasional dizziness, 25% patients suffered from nonspecific symptoms like mild headache, mental confusion and/or generalized convulsion as a result of mass effect, 13.9% of patients were seriously affected with marked debilitation, raised intracranial pressure, epileptic attacks and/or changes in consciousness level.
Imaging features
TLN lesions are usually restricted within the portals of radiation though they may extend well beyond. Enhanced computed tomography (CT) and magnetic resonance image (MRI) is commonly used in assessment of disease status. The characteristic manifestations of TLN on CT are finger like hypodense area which is representative of reactive white matter edema in early stage and cyst like changes corroborating with liquefactive necrosis and surrounding gliosis in late stage (28). While MRI is superior to CT in diagnosing TLN. The major performances of TLN on MRI (11) are (I) finger-like white-matter lesion on T2 weight image (T2WI), sometimes is accompanied with edema around necrotic foci, (II) contrast heterogeneous enhancement describe as Swiss cheese or soap bubble internally and spreading wave front marginally, (III) highly hyperintense well-defined cystic components on T2WI, (IV) sometimes mass effect could been observed as well. Figure 1 shows the representative MR images of a patients synchronously with TLN and recurrent lesions after radiation of NPC. Conventional imaging techniques are not really sufficient to diagnose all conditions. Advanced imaging tools like functional MRIs, positron emission tomography (PET) and single photon emission CT (SPECT) provide additional information and can be choose when there are indications.
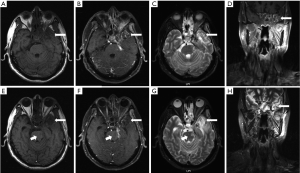
PWI allows a non-invasive evaluation of cerebral blood flow (CBF) and relative regional cerebral blood volume (rCBV). CBF, rCBV, mean transit time of contrast media (MTT) and time to peak (TTP) can be achieved in this sequence. Radiation necrosis was observed with very low CBV due to vascular damage (29). While tumors usually manifest a higher CBF and rCBV, lower MTT and TTP. It is reported in a study, a threshold of 406.5 mL for CBV and 29.5 mL/second for CBF was found to differentiate benign and malignant brain lesions with a sensitivity of 91% and specificity of 88% (30). Despite great diagnostic value, the potential pitfalls of PWI are its susceptibility to motion artifacts, relative but not absolute quantification of CBV and inaccurate determination of CBV in cases of severe disruption or absence of blood brain barrier (31). DWI quantitatively detects motions of water molecules in living tissue where water diffusion is anisotropic. TLN usually displays a marked high apparent diffuse coefficient (ADC) due to necrosis and a loosening of the extracellular space (32). Tumor with high cellularity, the added cell membrane mass impedes water movement and lead to a decrease in ADC (32). However, low ADC values have been observed in RT-induced cerebral necrosis in several studies, which they attributed to sterile liquefaction necrosis or a mixture of different components of radiation-induced necrosis (33-35). PWI and DWI provide information of hemodynamics and micro-structure/function. MR spectroscopy (MRS) as a non-invasive method, can be used to gain metabolic information, which help to characterize intracranial lesions. Lactate (Lac) and lipid (Lip) reflect anaerobic metabolism and cellular necrosis respectively, which is not detectable in healthy brain. Choline (Cho) represents cellular membrane phospholipid synthesis reflecting cell proliferation. N-acetyl-aspartate (NAA) functions as a neuronal integrity marker. Creatine (Cr) indicates cellular energy metabolism and is fairly stable under most conditions. Radiation necrosis generally marked by decrease in Cho, Cr and NAA (36), while malignancy usually presented with elevated Cho and decreased levels of Cr and NAA (37). Cho/NAA ratio is often used in brain MRS. A clinic decision model suggested patients with a Cho/NAA ratio <1.1 were assigned for imaging follow-up; those with a higher ratio >2.3 underwent immediate treatment in line with tumor; while patients with Cho/NAA ratios between these values would undergo biopsy (38). Another study suggested that complete absence of the Cho and NAA peaks, or progressive reduction of both accompanying with rising lipid and lactose peaks highly indicated radiation necrosis (39). However, MRS lacks the ability to precisely identify the boundaries of a tumor and radiation necrosis when they co-exist at the same location. And there is no consensus on the calculated threshold which can best distinguish radiation necrosis from a tumor. According to a meta-analysis, adding these functional MRIs to conventional radiological imaging can enhance diagnostic accuracy (40).
Nuclear medicine imaging techniques like PET and single-photon emission CT (SPECT) is based on the preferential uptake and retention of radiolabeled tracers by the target tissue. Tumor cells have a higher tendency to absorb these metabolites generating contrast in uptake between tumor mass and surrounding healthy tissue. Radiation necrosis with large amount of necrosis and fibrosis components, usually presents with low uptake activity. 18F-fluorodeoxyglucose (18F- FDG) is the most commonly tracers used in PET. Di Chiro et al. raised a question ‘Can PET-FDG challenge tumor histology?’ based on a large samples study indicating a 100% sensitivity and specificity with PET in the differentiation of tumor from radiation necrosis (41). However, this result was challenged by subsequent studies. A review indicated 18F-FDG PET might had less sensitivity of 81–86% due to recent RT, low histological grade and small tumor volume. While it had various range of specificity of 40–94%, false-positive cases happened in radiation-induced brain injury due to activated repair mechanisms or inflammatory activity (42). Other tracers such as 11C-methionine (MET), 18F-fluoroethyltyrosine (18F-FET), 18F-boronophenylalanine (18F-FBPA), 18F-fluorodihydroxyphenylalanine (18F-FDOPA) and 18F-fluorothymidine (FLT) have been going studied. In addition, hybrid PET/MRI provides not only the tracer uptake PET imaging but also combines morphological and functional MRI. It theoretically seems to have the highest diagnostic accuracy (43,44). The application of above technique remains to be specifically proved by clinical studies. The disadvantage of PET is that it is expensive, not widely available and ironizing radiation. In term of SPECT, the common tracers used are 201Thallium (Tl), 99mTcchnetium-methoxyisobutylisonitrile (Tc-MIBI), 99mTc-glucoheptonate (GHA). Yamamoto et al. (45) reported that 201Tl and 99mTc-MIBI concentration was significantly higher in recurrent tumor than brain necrosis, with accuracy of 90% to diagnosis for radiation necrosis in both tracers, and concentration level was higher in 99mTc-MIBI than 201Tl. When compare to PET it is a less costly method, however the spatial resolution is slightly low.
Advanced image techniques are promising, meanwhile further prospective studies are required to clearly establish the clinical usefulness of them.
Diagnosis and differential diagnosis
Absolutely, pathological biopsy is the golden standard for the diagnosis of TLN. However, due to the limitation of intracranial lesion sampling, this invasive procedure is rare adopted. In many cases a working diagnosis can still be reached without resorting to biopsy. To carefully consider symptoms, radiation history, latency after radiation, reviewing the treatment plan (radiation necrosis usually locates in region with relative highly biological effective dose), image features and tumor biomarkers.
Differential diagnosis of TLN includes intracranial extension of NPC, primary intracranial tumors, second intracranial malignancies, hematogenous cerebral metastasis and brain abscess. It is easier to exclude brain abscess on the basis of symptoms and laboratory investigations suggestive of infection. Hematogenous cerebral metastasis from NPC are extremely rare (46). If temporal lobe lesion with patchy enhancement continues with cavernous sinus where was invaded by tumor when newly diagnosed, intracranial extension of NPC can’t be excluded. At this point, it’s necessary to combine multiple image examination and copy number of circulating plasma EBV DNA. Most radiation-induced necrosis presents as an isolated lesion in the temporal lobe locating in radiation area. Primary tumors like glioma, lymphoma and radiation-induced sarcoma should be excluded by comprehensively considering epidemic, clinical and image features.
Management
No consensus on the management for TLN secondary to radiotherapy for NPC has been established. Prevention remains the most effective method in the management of TLN, like using advanced radiation techniques, optimizing radiation plan, etc. Reduction of TLN risk needs to be balanced with tumor control. Once TLN happens, for asymptomatic TLN, observation with close image follow-up is generally suggested. For those with symptomatic TLN, active treatment should be given, the clinical goals are to reduce symptoms and improve quality of life. Interventions for TLN are developing, the treatment modalities available, both established and experimental, are as follows.
Corticosteroids
Corticosteroids are the classic treatment for symptomatic TLN. It can reduce inflammatory response, alleviated edema and relieve symptoms. Methylprednisolone and dexamethasone are commonly used in clinical practice, proton pump inhibitors are prophylactically used together with them in order to avoid gastric ulcer and bleed. Lam et al. (27) demonstrated that intravenously pulsed steroid was associated with better clinical response than oral steroid delivery. Corticosteroids seem to be beneficial only in early phase of liquefactive necrosis. The value of corticosteroids is symptom relief rather than cure. Prolonged use of steroids is related to myopathy, weight gain, and even infection duo to immunosuppressive effects. In a clinical trial, the response rate is 31.5% by using methylprednisolone alone (47). Thus, it’s crucial to identify steroid refractory TLN. The combination of corticosteroids with other effective agents present a better control of TLN in several studies (48,49).
Anticoagulation & antiplatelet
Anticoagulants reduce platelet aggregation and prevent thrombogenesis, which can inhibit one of formation mechanisms in TLN. The use of heparin and warfarin in the treatment of cerebral radiation necrosis is reported in literature (50). In this small case series patients experienced relief in a short-term, whereas symptoms recurrence after discontinuation. The safety of this treatment is questionable as a risk of hemorrhage. There is a lack of large randomized controlled studies to assess the safety and the benefits of anticoagulant therapy.
Bevacizumab (BEV)
BEV is a humanized murine monoclonal antibody against VEGF. In 2007, it was first reported with ideal effect in radiation-induced necrosis (51). Since then, an emerging body of studies have examined the use of this agent in the treatment of radiation necrosis, most of them are case reports, case series, pilot studies and retrospective studies with small sample size and only 2 randomized clinical trails were performed (47,52-61). Levin et al. (56) compared BEV (7 patient: 7.5 mg/kg) to placebo (7 patient: saline) every 3 weeks, 4 cycles in the treatment of radiation necrosis, and showed good efficacy of BEV in radiation necrosis. Patients in BEV group were observed with significant decreases in lesion volume. Seven patients initially randomized to placebo then crossed over to receive BEV as they had obvious progression during follow-up, then showed improvements in neurological signs and symptoms after using BEV. Randomized controlled trials (RCT) conducted by Xu et al. (47), demonstrated that BEV offers improved symptomatic relief and radiographic response over corticosteroids. The side effects such as hypertension, proteinuria and thrombosis were mild and manageable. According to above results, BEV positively alleviate symptom, improve radiographic signs and reduce dosage of steroids in radiation necrosis. However, follow-up of the studies is not long enough, and many studies reported recurrence of radiation necrosis following BEV discontinuation or even along with BEV treatment (62-64). Most of patients initially responded to BEV, but with prolonged treatment their condition deteriorated. Researchers considered it might attribute to the phenomenon of over-pruning. Over inhibition of VEGF break the balance of angiogenesis and anti-angiogenesis. Other recurred patients after discontinuation of BEV suggests that BEV is effective in reducing edema, but it still can’t reverse radiation necrosis by BEV. Currently, no uniform established standard regarding the application of BEV. There are still many questions. Firstly, how to screen patients who are sensitive to BEV? BEV is costly and some patients (about 34%) failed to benefit from it (47). A study by Li et al. (65) explored predictive factors of therapeutic effects of BEV in NPC patients with radiation-induced TLN. The results suggested that BEV might be more effective in patients with a lower maximum radiation dose to TL, however, no cutoff value was found in this study, further study warrants to be done in the future. Secondly, what is the optimal dose? Various dosages range from 5 mg/kg to15 mg/kg were used in previous studies, a low dose of 5–7.5 mg/kg was reported to have satisfactory effect (47,51,56,60,66). A small sample size retrospective study (59) demonstrated that no difference in clinical and radiologic outcomes when high-dose (10–15 mg/kg) was compared with low-dose (5–7.5 mg/kg). Thirdly, what is the best treatment course? Researchers thought there might be a BEV resistance caused by over-pruning, it’s better to give short-course, stop timely upon obtaining satisfactory effects and reuse if radiation necrosis progresses.
Nerve growth factor (NGF)
In the past decades, amounts of studies demonstrated that glial and neuron damage is associated with the development of TLN. It helps us to explore new agents in the treatment for TLN. NGF is a growth factor and neuropeptide primarily involved in the regulation of growth, maintenance, proliferation and survival of certain target neurons both in peripheral and central nervous systems. In 2014, Wang et al. firstly reported that mouse nerve growth factor has the potential to reverse TLN (67). Consequently, a phase II trial conducted by them, comparing the combination of corticosteroids and NGF (study group) with corticosteroids alone (control group) in the treatment of TLN, both objective and subjective evaluation showed it was significantly better in the study group than the control group (48). Moreover, three patients had complete response. Hence, the researcher speculated that the process of TLN was not irreversible. Whereas, routine clinical practice has to base on high-ranking evidence like meta-analysis or well-designed phase III clinical trials. The role of nerve growth factor needs to be further validated. It must be emphasized that NGF is a growth factor, whether it will promote tumor stem cell growth is still uncertain. Hence, it must be careful to rule out tumor recurrence or metastasis when enrolling patients in this treatment.
Gangliosides
Gangliosides are sphingolipids containing sialic acid. They are cell membrane components, predominantly in the central nervous system. Monosialotetrahexosylganglioside (GM1), the prototype ganglioside, is a member of gangliosides which contains one sialic acid residue. GM1 is involved in the development of nervous system and has neurotropic and neuroprotective functions. GM1 is efficacious in animal models of neurodegenerative disease (68). Pathological processes such as ischemia, hypoxia are often accompanied with decrease level of ganglioside. Certain amount supplement of GM1 can prevent neuron apoptosis, axonal damage and improve the ability of learning and memory. Clinical experience is richest with GM1 in the treatment of stroke. Fourteen RCTs consistently reported superior neurological outcomes when compared to placebo, although no difference in survival was found (69). Two RCTs in Parkinson’s disease provided evidence of GM1 to be superior to placebo in improving motor symptoms and slowing progression (70,71). GM1 would be a welcome therapy in neurodegenerative disease with promising prospects. The application of GM1 in cerebral necrosis has not been reported in literatures. Some researcher speculated that GM1 might have a potential therapeutic effect in cerebral radiation necrosis. A randomized clinical trial (NCT03067753) comparing the efficacy of GM1 and methylprednisolone is undergoing in China. We are looking forward the results of this study.
Edaravone
Edaravone is a free radical scavenger, which can help to eliminate ROS in the pathogenesis of radiation-induced brain injury. A RCT of 154 patients showed that significantly more patients in the study group using edaravone and steroid regimen achieved edema decreases ≥25% than that in the steroid alone group (55.6% vs. 35.4%) (49). Based on the mechanism of edaravone, it might play a beneficial role in prophylactic treatment, however, whether it will against the treatment of tumor therapy is unclear, which needs to be further studied.
Surgery
Surgery as an invasive method, is an alternative but not preferred treatment (72). Resection is reserved for medical therapy refractory TLN or in situations in which the diagnosis is unclear. Moreover, with an emergency of intracranial hypertension, concurrence of intracranial hemorrhage, etc. surgery is required to provide a beneficial palliative effect.
In addition to above treatments, vitamin E and pentoxifylline (73,74), hyperbaric oxygen therapy (75) and laser interstitial thermal ablation (76) can also be considered in the treatment of TLN. However, benefit of these treatment modalities is not well established and no RCT was published in literatures. It is also reported that exogenous neural stem cells supplementation can prevent radiation-induced functional loss of the brain in animal model (77), suggesting it has the potential to prevent the occurrence of TLN.
Conclusions
In conclusion, therapeutic options for the treatment of cerebral radiation necrosis are increasing, such as bevacizumab, NGF, GM1, edaravone. However, large, randomized controlled researches with long-term follow-up trial or meta-analysis is needed to evaluation the safety and confirm the efficacy on these treatments.
Acknowledgments
Funding: This study was funded by grant from National Natural Science Foundation of China (No. 81672971).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Imjai Chitapanarux) for the series “Late Complications in the Management of Nasopharyngeal Cancer” published in Annals of Nasopharynx Cancer. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at http://dx.doi.org/10.21037/anpc-2019-LCMNC-03
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/anpc-2019-LCMNC-03). The series “Late Complications in the Management of Nasopharyngeal Cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Chen YP, Chan ATC, Le QT, et al. Nasopharyngeal carcinoma. Lancet 2019;394:64-80. [Crossref] [PubMed]
- Su SF, Huang SM, Han F, et al. Analysis of dosimetric factors associated with temporal lobe necrosis (TLN) in patients with nasopharyngeal carcinoma (NPC) after intensity modulated radiotherapy. Radiat Oncol 2013;8:17. [Crossref] [PubMed]
- Peng G, Wang T, Yang KY, et al. A prospective, randomized study comparing outcomes and toxicities of intensity-modulated radiotherapy vs. conventional two-dimensional radiotherapy for the treatment of nasopharyngeal carcinoma. Radiother Oncol 2012;104:286-93. [Crossref] [PubMed]
- Leung SF, Kreel L, Tsao SY. Asymptomatic temporal lobe injury after radiotherapy for nasopharyngeal carcinoma: Incidence and determinants. Br J Radiol 1992;65:710-4. [Crossref] [PubMed]
- Blanchard P, Lee A, Marguet S, et al. Chemotherapy and radiotherapy in nasopharyngeal carcinoma: an update of the MAC-NPC meta- analysis. Lancet Oncol 2015;16:645. [Crossref] [PubMed]
- Guo Q, Lu T, Lin S, et al. Long-term survival of nasopharyngeal carcinoma patients with Stage II in intensity-modulated radiation therapy era. Jpn J Clin Oncol 2016;46:241-7. [Crossref] [PubMed]
- Chen Y, Sun Y, Liang SB, et al. Progress report of a randomized trial comparing long-term survival and late toxicity of concurrent chemoradiotherapy with adjuvant chemotherapy versus radiotherapy alone in patients with stage III to IVB nasopharyngeal carcinoma from endemic regions of China. Cancer 2013;119:2230-8. [Crossref] [PubMed]
- Lee AWM, Foo W, Chappell R, et al. Effect of time, dose, and fractionation on temporal lobe necrosis following radiotherapy for nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 1998;40:35-42. [Crossref] [PubMed]
- Zhou GQ, Yu XL, Chen M, et al. Radiation-Induced Temporal Lobe Injury for Nasopharyngeal Carcinoma: A Comparison of Intensity-Modulated Radiotherapy and Conventional Two-Dimensional Radiotherapy. PLoS One 2013;8:e67488 [Crossref] [PubMed]
- Mao YP, Zhou GQ, Liu LZ, et al. Comparison of radiological and clinical features of temporal lobe necrosis in nasopharyngeal carcinoma patients treated with 2D radiotherapy or intensity-modulated radiotherapy. Br J Cancer 2014;110:2633-9. [Crossref] [PubMed]
- Kim TH, Ko YH, Lee MA, et al. Treatment Outcome of Cisplatin-based Concurrent Chemoradiotherapy in the Patients with Locally Advanced Nasopharyngeal Cancer. Cancer Res Treat 2008;40:62. [Crossref] [PubMed]
- Sun Y, Li WF, Chen NY, et al. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase 3, multicentre, randomised controlled trial. Lancet Oncol 2016;17:1509-20. [Crossref] [PubMed]
- Rahmathulla G, Marko NF, Weil RJ. Cerebral radiation necrosis: A review of the pathobiology, diagnosis and management considerations. J Clin Neurosci 2013;20:485-502. [Crossref] [PubMed]
- Edwards MS, Levin VA, Byrd A. Quantitative observations of the subacute effects of X irradiation on brain capillary permeability: Part I. Int J Radiat Oncol Biol Phys 1979;5:1633-5. [Crossref] [PubMed]
- Nordal RA, Nagy A, Pintilie M, et al. Hypoxia and hypoxia-inducible factor-1 target genes in central nervous system radiation injury: A role for vascular endothelial growth factor. Clin Cancer Res 2004;10:3342-53. [Crossref] [PubMed]
- Yoritsune E, Furuse M, Kuwabara H, et al. Inflammation as well as angiogenesis may participate in the pathophysiology of brain radiation necrosis. J Radiat Res 2014;55:803-11. [Crossref] [PubMed]
- Fike JR, Rosi S, Limoli CL. Neural Precursor Cells and CNS Radiation Sensitivity. Semin Radiat Oncol 2009;19:122-32. [Crossref] [PubMed]
- Koot RW, Stalpers LJA, Aronica E, et al. Cerebral necrosis after 25 Gy radiotherapy in childhood followed 28 years later by 54 Gy radiotherapy. Clin Neurol Neurosurg 2007;109:607-12. [Crossref] [PubMed]
- Su SF, Huang Y, Xiao WW, et al. Clinical and dosimetric characteristics of temporal lobe injury following intensity modulated radiotherapy of nasopharyngeal carcinoma. Radiother Oncol 2012;104:312-6. [Crossref] [PubMed]
- Huang J, Kong FF, Oei RW, et al. Dosimetric predictors of temporal lobe injury after intensity-modulated radiotherapy for T4 nasopharyngeal carcinoma: A competing risk study. Radiat Oncol 2019;14:31. [Crossref] [PubMed]
- Wang L, Hu J, Liu X, et al. Intensity-modulated carbon-ion radiation therapy versus intensity-modulated photon-based radiation therapy in locally recurrent nasopharyngeal carcinoma: A dosimetric comparison. Cancer Manag Res 2019;11:7767-77. [Crossref] [PubMed]
- Lee AW, Ng WT, Hung WM, et al. Major Late Toxicities After Conformal Radiotherapy for Nasopharyngeal Carcinoma-Patient- And Treatment-Related Risk Factors. Int J Radiat Oncol Biol Phys 2009;73:1121-8. [Crossref] [PubMed]
- Wang J, Miao Y, Ou X, et al. Development and validation of a model for temporal lobe necrosis for nasopharyngeal carcinoma patients with intensity modulated radiation therapy. Radiat Oncol 2019;14:42. [Crossref] [PubMed]
- Niu X, Hu C, Kong L. Experience with combination of cetuximab plus intensity-modulated radiotherapy with or without chemotherapy for locoregionally advanced nasopharyngeal carcinoma. J Cancer Res Clin Oncol 2013;139:1063-71. [Crossref] [PubMed]
- Wang TM, Shen G, Chen M, et al. Genome-Wide Association Study of Susceptibility Loci for Radiation-Induced Brain Injury J Natl Cancer Inst 2019;111:620-8. [Crossref] [PubMed]
- Lam TC, Wong FC, Leung TW, et al. Clinical Outcomes of 174 Nasopharyngeal Carcinoma Patients With Radiation-Induced Temporal Lobe Necrosis. Int J Radiat Oncol Biol Phys 2012;82:e57-65. [Crossref] [PubMed]
- Lee AW, Ng SH, Ho JH, et al. Clinical Diagnosis of Late Temporal Lobe Necrosis Following Radiation Therapy for Nasopharyngeal Carcinoma. Cancer. 1988;61:1535-42. [Crossref] [PubMed]
- Larsen VA, Simonsen HJ, Law I, et al. Evaluation of dynamic contrast-enhanced T1-weighted perfusion MRI in the differentiation of tumor recurrence from radiation necrosis. Neuroradiology 2013;55:361-9. [Crossref] [PubMed]
- Aydın ZB, Aydın H, Birgi E, et al. Diagnostic Value of Diffusion-weighted Magnetic Resonance (MR) Imaging, MR Perfusion, and MR Spectroscopy in Addition to Conventional MR Imaging in Intracranial Space-occupying Lesions. Cureus 2019;11:e6409 [PubMed]
- Cha S, Knopp EA, Johnson G, et al. Intracranial Mass Lesions: Dynamic Contrast-Enhanced Susceptibility-Weighted Echo-Planar Perfusion MR Imaging. Radiology 2002;223:11-29. [Crossref] [PubMed]
- Chan YL, Yeung DKW, Leung SF, et al. Diffusion-weighted magnetic resonance imaging in radiation-induced cerebral necrosis: Apparent diffusion coefficient in lesion components. J Comput Assist Tomogr 2003;27:674-80. [Crossref] [PubMed]
- Tung GA, Evangelista P, Rogg JM, et al. Diffusion-weighted MR Imaging of Rim-Enhancing Brain Masses: Is Markedly Decreased Water Diffusion Specific for Brain Abscess? AJR Am J Roentgenol 2001;177:709-12. [Crossref] [PubMed]
- Le Bihan D, Turner R, Douek P, et al. Diffusion MR Imaging: Clinical Applications. AJR Am J Roentgenol 1992;159:591-9. [Crossref] [PubMed]
- Le Bihan D, Douek P, Argyropoulou M, et al. Diffusion and perfusion magnetic resonance imaging in brain tumors. Top Magn Reson Imaging 1993;5:25-31. [Crossref] [PubMed]
- Kozić D, Ostojić J, Bjelan M, et al. The Role of MR Spectroscopy in Neurooncology. Prilozi 2012;33:425-33. [PubMed]
- Möller-Hartmann W, Herminghaus S, Krings T, et al. Clinical application of proton magnetic resonance spectroscopy in the diagnosis of intracranial mass lesions. Neuroradiology 2002;44:371-81. [Crossref] [PubMed]
- Smith EA, Carlos RC, Junck LR, et al. Developing a clinical decision model: MR spectroscopy to differentiate between recurrent tumor and radiation change in patients with new contrast-enhancing lesions. AJR Am J Roentgenol 2009;192:W45-52 [Crossref] [PubMed]
- De Stefano N, Mortilla M, Federico A. Proton magnetic resonance spectroscopy of the brain in dementia. Ital J Neurol Sci 1999;20:S258-64. [Crossref] [PubMed]
- Furuse M, Nonoguchi N, Yamada K, et al. Radiological diagnosis of brain radiation necrosis after cranial irradiation for brain tumor: A systematic review. Radiat Oncol 2019;14:28. [Crossref] [PubMed]
- Di Chiro G, Fulham MJ. Virchow’s shackles: can PET-FDG challenge tumor histology? AJNR Am J Neuroradiol 1993;14:524-7. [PubMed]
- Langleben DD, Segall GM. PET in differentiation of recurrent brain tumor from radiation injury. J Nucl Med 2000;41:1861-7. [PubMed]
- Miller-Thomas MM, Benzinger TL. Neurologic Applications of PET/MR Imaging. Magn Reson Imaging Clin N Am 2017;25:297-313. [Crossref] [PubMed]
- Leiva-Salinas C, Muttikkal TJE, Flors L, et al. FDG PET/MRI Coregistration Helps Predict Response to Gamma Knife Radiosurgery in Patients With Brain Metastases. AJR Am J Roentgenol 2019;212:425-30. [Crossref] [PubMed]
- Yamamoto Y, Nishiyama Y, Toyama Y, et al. Tc-MIBI and 201 Tl SPET in the detection of recurrent brain tumours after radiation therapy. Nucl Med Commun 2002;23:1183-90. [Crossref] [PubMed]
- Dassarath M, Yin Z, Chen J, et al. Temporal lobe necrosis: A dwindling entity in a patient with nasopharyngeal cancer after radiation therapy. Head Neck Oncol 2011;3:8. [Crossref] [PubMed]
- Xu Y, Rong X, Hu W, et al. Bevacizumab Monotherapy Reduces Radiation-induced Brain Necrosis in Nasopharyngeal Carcinoma Patients: A Randomized Controlled Trial. Int J Radiat Oncol Biol Phys 2018;101:1087-95. [Crossref] [PubMed]
- Wang XS, Ying HM, He XY, et al. Treatment of cerebral radiation necrosis with nerve growth factor: A prospective, randomized, controlled phase II study. Radiother Oncol 2016;120:69-75. [Crossref] [PubMed]
- Tang Y, Rong X, Hu W, et al. Effect of edaravone on radiation-induced brain necrosis in patients with nasopharyngeal carcinoma after radiotherapy: a randomized controlled trial. J Neurooncol 2014;120:441-7. [Crossref] [PubMed]
- Glantz MJ, Burger PC, Friedman AH, et al. Treatment of radiation-induced nervous system injury with heparin and warfarin. Neurology 1994;44:2020-7. [Crossref] [PubMed]
- Gonzalez J, Kumar AJ, Conrad CA, et al. Effect of bevacizumab on radiation necrosis of the brain. Int J Radiat Oncol Biol Phys 2007;67:323-6. [Crossref] [PubMed]
- Wong ET, Huberman M, Lu XQ, et al. Bevacizumab reverses cerebral radiation necrosis. J Clin Oncol 2008;26:5649-50. [Crossref] [PubMed]
- Dahl NA, Liu AK, Foreman NK, et al. Bevacizumab in the treatment of radiation injury for children with central nervous system tumors. Childs Nerv Syst 2019;35:2043-6. [Crossref] [PubMed]
- Liu AK, Macy ME, Foreman NK. Bevacizumab as therapy for radiation necrosis in four children with pontine gliomas. Int J Radiat Oncol Biol Phys 2009;75:1148-54. [Crossref] [PubMed]
- Torcuator R, Zuniga R, Mohan YS, et al. Initial experience with bevacizumab treatment for biopsy confirmed cerebral radiation necrosis. J Neurooncol 2009;94:63-8. [Crossref] [PubMed]
- Levin VA, Bidaut L, Hou P, et al. Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys 2011;79:1487-95. [Crossref] [PubMed]
- Wang Y, Pan L, Sheng X, et al. Reversal of cerebral radiation necrosis with bevacizumab treatment in 17 Chinese patients. Eur J Med Res 2012;17:25. [Crossref] [PubMed]
- Yonezawa S, Miwa K, Shinoda J, et al. Bevacizumab treatment leads to observable morphological and metabolic changes in brain radiation necrosis. J Neurooncol 2014;119:101-9. [Crossref] [PubMed]
- Sadraei NH, Dahiya S, Chao ST, et al. Treatment of Cerebral Radiation Necrosis With Bevacizumab. Am J Clin Oncol 2015;38:304-10. [Crossref] [PubMed]
- Hu Q, Zhao J, Xu J, et al. Long-Term Relief of Cerebral Radiation Necrosis Treated with Low-Dose Bevacizumab-a Report of 2 Cases. Oncol Res Treat 2017;40:133-7. [Crossref] [PubMed]
- Meng X, Zhao R, Wu S, et al. Efficacy of repeated low-dose bevacizumab treatment with long-dosing interval for radiation-induced brain necrosis: A case report. Cancer Biol Ther 2017;18:63-6. [Crossref] [PubMed]
- Zhuang H, Yuan X, Sun D, et al. Acquired-resistance of bevacizumab treatment for radiation brain necrosis: a case report. Oncotarget 2016;7:13265-8. [Crossref] [PubMed]
- Zhuang H, Yuan X, Chang JY, et al. Exploration of the recurrence in radiation brain necrosis after bevacizumab discontinuation. Oncotarget 2016;7:48842-9. [Crossref] [PubMed]
- Jeyaretna DS, Curry WT, Batchelor TT, et al. Exacerbation of cerebral radiation necrosis by bevacizumab. J Clin Oncol 2011;29:e159-62. [Crossref] [PubMed]
- Li Y, Huang X, Jiang J, et al. Clinical Variables for Prediction of the Therapeutic Effects of Bevacizumab Monotherapy in Nasopharyngeal Carcinoma Patients with Radiation-induced Brain Necrosis. Int J Radiat Oncol Biol Phys 2018;100:621-9. [Crossref] [PubMed]
- Alessandretti M, Buzaid AC, Brandão R, et al. Low-Dose Bevacizumab Is Effective in Radiation-Induced Necrosis. Case Rep Oncol 2013;6:598-601. [Crossref] [PubMed]
- Wang X, Ying H, Zhou Z, et al. Successful treatment of Radiation-Induced Temporal Lobe Necrosis With Mouse Nerve Growth Factor. J Clin Oncol 2011;29:e166-8. [Crossref] [PubMed]
- Ramirez MR, Muraro F, Zylbersztejn DS, et al. Neonatal hypoxia-ischemia reduces ganglioside, phospholipid and cholesterol contents in the rat hippocampus. Neurosci Res 2003;46:339-47. [Crossref] [PubMed]
- Magistretti PJ, Geisler FH, Schneider JS, et al. Gangliosides: Treatment avenues in neurodegenerative disease. Front Neurol 2019;10:859. [Crossref] [PubMed]
- Schneider JS, Gollomp SM, Sendek S, et al. A randomized, controlled, delayed start trial of GM1 ganglioside in treated Parkinson’s disease patients. J Neurol Sci 2013;324:140-8. [Crossref] [PubMed]
- Schneider JS, Roeltgen DP, Mancall EL, et al. Parkinson’s disease improved function with GM1 ganglioside treatment in a randomized placebo-controlled study. Neurology 1998;50:1630-6. [Crossref] [PubMed]
- McPherson CM, Warnick RE. Results of contemporary surgical management of radiation necrosis using frameless stereotaxis and intraoperative magnetic resonance imaging. J Neurooncol 2004;68:41-7. [Crossref] [PubMed]
- Williamson R, Kondziolka D, Kanaan H, et al. Adverse Radiation Effects after Radiosurgery May Benefit from Oral Vitamin E and Pentoxifylline Therapy: A Pilot Study. Stereotact Funct Neurosurg 2008;86:359-66. [Crossref] [PubMed]
- Chan AS, Cheung MC, Law SC, et al. Phase II study of alpha-tocopherol in improving the cognitive function of patients with temporal lobe radionecrosis. Cancer 2004;100:398-404. [Crossref] [PubMed]
- Chuba PJ, Aronin P, Bhambhani K, et al. Hyperbaric Oxygen Therapy for Radiation-Induced Brain Injury in Children. Cancer 1997;80:2005-12. [Crossref] [PubMed]
- Rahmathulla G, Recinos PF, Valerio JE, et al. Laser Interstitial Thermal Therapy for Focal Cerebral Radiation Necrosis: A Case Report and Literature Review. Stereotact Funct Neurosurg 2012;90:192-200. [Crossref] [PubMed]
- Joo KM, Jin J, Kang BG, et al. Trans-differentiation of neural stem cells: A therapeutic mechanism against the radiation induced brain damage. PLoS One 2012;7:e25936 [Crossref] [PubMed]
Cite this article as: Li PJ, Chen YY. Narrative review: temporal lobe necrosis after radiotherapy for nasopharyngeal carcinoma. Ann Nasopharynx Cancer 2020;4:10.


