
Biological opportunities for personalized radiotherapy of nasopharyngeal carcinoma
Introduction
Nasopharyngeal carcinoma (NPC) is highly sensitive to ionizing radiation, and radiotherapy is the mainstay treatment modality for nonmetastatic disease. Modern techniques of radiotherapy, such as intensity modulated radiotherapy (IMRT) and image guided radiotherapy (IGRT), have allowed accurate delivery of radiation dose based on anatomical and geometrical information, which provides improved tumor target coverage with significantly better sparing of normal tissues (1,2). The advancement of radiation techniques has led to substantial improvement of locoregional tumor control (3,4), as well as decreased radiation-induced toxicities for nonmetastatic NPC (5). Despite this, radiotherapy of NPC has not yet entered the era of precision medicine, in which radiotherapy should be tailored to individual patients based on the biological features.
In oncology, genomics has been gradually integrated into the routine clinical practice to guide personalized chemotherapy and targeted therapy in many cancers (6,7). However, personalized radiotherapy remains largely uninvestigated. A good example comes from the pilot attempts to reduce radiation dose in oropharyngeal cancer positive for human papillomavirus (HPV) since the higher radiosensitivity of HPV-positive oropharyngeal cancer due to lower DNA damage repair capacity (8,9). Furthermore, an important concept relating personalized radiotherapy was introduced as genomic-adjusted radiation dose by Scott et al. (10). They developed a genome-based model to derive an optimum genomic-adjusted radiation dose, which allow the individualization of radiotherapy dose to tumor radiosensitivity for different cancers including head and neck squamous cell carcinoma (HNSCC). For example, a high genomic-adjusted radiation dose suggests of high therapeutic effect of radiotherapy, which may indicate de-escalation of the radiation dose. This study is the first to develop a strategy to integrate biological differences of radiosensitivity into radiotherapy dose and it suggests that biology-driven personalize strategies would be a promising approach to further widening of the therapeutic window of radiotherapy.
In terms of NPC, personalized radiotherapy is actually an under-researched scope. In this review, with the purpose of promoting the development of novel biology-driven strategies for personalized radiotherapy of NPC, we discussed the so far evidence on current clinical practice relating personalized radiotherapy, and biological mechanisms of radioresistance of NPC and targeting the mechanisms to enhance radiosensitivity.
Current clinical practice relating personalized radiotherapy of NPC
Risk stratification of tumor recurrence in NPC
At present, risk-stratified therapy is an effective way to tailor personalized and precise treatment to NPC, especially in combined chemotherapy. For Radiotherapy, a retrospective study demonstrated that a moderately reduced of radiation dose by about 10% delivered with IMRT resulted in comparable prognosis to those with prescription dose of 70 Gy in patients stratified by tumor stage; thus, indicating the effectiveness of de-escalated radiation dose in patients of T1–T3 NPC (11). However, this needs to be validated in a prospective study with a larger sample size and more precise risk stratification is in urgent need.
Other than tumor-node-metastasis (TNM) staging system, lots of studies have proposed different clinical or radiomics models and biomarkers for more precise risk stratification of tumor recurrence in NPC (12-14). In patients with non-metastatic NPC who received radical IMRT, Chen et al. developed pretreatment nomograms, by incorporating clinical and pathological features of the tumors, to predict the risk of local and regional recurrence (12). These nomograms shown more accurate risk stratification performance than TNM staging system. Focused on patients with the most locally advanced NPC (T4 classification), radiomics signature from multiparametric magnetic resonance (MR) imaging was introduced to further improved the performance of clinical nomogram (13). Although these validated models could achieve better risk stratification of tumor recurrence in NPC, the biology-based stratification of radiosensitivity of NPC is urgently needed to guide escalation or de-escalation of radiation dose in tumors with distinct radiosensitivity.
Dose escalation guided by functional imaging
In terms of radiation dose escalation, a pilot prospective study has evaluated the effect and toxicities of fluorine-18 fluorodeoxyglucose (18F-FDG) positron emission tomography/computed tomography (PET/CT)-guided dose painting IMRT (DP-IMRT), which could identify an appropriate tumor volume to be prescribed a higher radiation dose in locoregionally advanced NPC (15). In DP-IMRT group, a higher dose (75.2 Gy in 32 and 77.55 Gy in 33 fractions for patients with T1–2 and T3–4 disease, respectively) was prescribed to the subvolumes defined by the isocontour of maximum 50% standard uptake value (SUV); while the prescribed dose in CT-based IMRT group was 70.4–72.6 Gy in 32–33 fractions. Results showed that DP-IMRT significantly improved 3-year local failure-free survival (98.8% vs. 91.3%; P=0.032), locoregional failure-free survival (97.2% vs. 91.2%; P=0.049), and overall survival (91.8% vs. 82.6%; P=0.049). No statistically significant differences in acute and late toxic effects were observed. The results indicated that 18F-FDG PET/CT or other functional imaging might phenotype tumor heterogeneity of NPC, hence DP-IMRT guided by functional imaging might be a way to achieve personalized radiotherapy.
Additionally, emerging radiation technologies like proton therapy and carbon ion therapy have become available, offering new opportunities for escalation of radiation dose, improving tumor control and reducing toxicities (16,17), due to the physical characteristics that allow delivery of a high radiation dose to the tumor and maximal sparing of surrounding normal tissues. At present, these techniques are mainly studied in recurrent NPC. In the future, with the purpose of personalized radiotherapy, the right patients should be identified by biomarkers or functional imaging for dose escalation using proton therapy and carbon ion therapy at their primary treatment.
Targeting mechanisms of radioresistance in NPC
Radioresistance remains a major cause of local and regional recurrence in NPC patients; thus, predicting and targeting radioresistance could provide more personalized and effective strategies for radiotherapy and further improve the outcomes of NPC patients. To date, many studies have investigated the mechanisms of radioresistance, such as DNA damage repair, tumor hypoxia and autophagy. Figure 1 demonstrates the example mechanisms involving in radioresistance of NPC and their clinical implications. Identifying and targeting the biomarkers could help to enhance the radiosensitivity of NPC and guide biology-driven personalized radiotherapy.
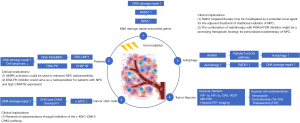
DNA damage repair
The most devastating damage of ionizing radiation is DNA double-strand breaks (DSBs), which induce cell-cycle arrest or cell death (18). Many studies have attempted to identify the molecules involved in DNA damage repair. For example, several DNA damage repair-associated genes were found to be significantly differed between radioresistant and radiosensitive NPC biopsy samples, of which replication protein A3 (RPA3) has been identified as a candidate radioresistance biomarker that associated with poor prognosis (19). Given that RPA3 was found to correlate only with radiosensitivity but not metastasis, it may have an advantage and potential application in guiding personalized radiotherapy of NPC (19). Besides, it also observed in this study that TP53 was significantly down-regulated in radioresistant NPC samples. Historical data have confirmed that TP53-mediated apoptosis may play an important role in the TP53-associated radio-sensitization (20), and decreased TP53 expression could enhance the radioresistance of some solid tumors (21,22).
Importantly, some proteins were found to enhance the ability of DNA damage repair and induce radioresistance of tumor cells. Lu and colleagues revealed that Epstein-Barr virus (EBV) encoded latent membrane protein 1 (EBV-LMP1) could suppress the DNA damage response through DNA-dependent protein kinase (DNA-PK)/adenosine 5’-monophosphate (AMP)-activated protein kinase (AMPK) signaling to promote radioresistance in NPC (23), which provided a mechanistic rationale in supporting the use of AMPK activators for NPC radiosensitization. Another study shown that chromatin assembly factor 1 subunit B (CHAF1B) enhances radioresistance by promoting DNA damage repair and inhibiting cell apoptosis, also in a DNA-PK pathway-dependent way, and indicated that DNA-PK inhibitor could serve as a radiosensitizer for patients with NPC and high CHAF1B expression (24).
Additionally, studies have suggested that cancer stem cells (CSCs) are more resistant to chemotherapy and radiotherapy than non-stem cells, mainly due to their differential response to DNA damage (25). In NPC, a recent study showed that overexpression of proto-oncogene c-MYC in CSCs contribute to radioresistance through preferential activation of the checkpoint kinase-1 (CHK1) and checkpoint kinase-2 (CHK2) checkpoint response and an increase in DNA damage repair capacity (26). Furthermore, loss of CHK1 and CHK2 expression would enhance radiosensitivity in vitro and in vivo. This study elucidates the role of the c-MYC-CHK1/CHK2 axis in regulating DNA damage checkpoint responses and reveals a potential therapeutic application in reversal of radioresistance through inhibition of the c-MYC-CHK1/CHK2 pathway.
Other than coding genes and proteins, non-coding ribonucleic acids (RNAs), such as micro RNAs (miRNA) and long non-coding RNAs (lncRNAs) also play a sizable role in radioresistance of NPC (27). Specifically, some miRNAs promote NPC radioresistance by targeting cell signaling pathways to promote DNA damage repair (28,29). Conversely, some other miRNAs could reduce NPC radioresistance (30). lncRNAs are also proved to have effects on radioresistance of NPC by involving in DNA damage repair, cell proliferation and apoptosis (31,32). Targeting radioresistance mechanisms of these non-coding RNAs might also be a potential strategy for personalized radiotherapy of NPC.
Despite uncovering mechanisms and biomarkers of radiosensitivity in NPC, which could be used to identify patients who could benefit from a biology-driven personalized treatment, as well as serve as new therapeutic targets for radiosensitization, the clinical use of these biomarkers remains substantially limited and warrant further investigation.
Tumor hypoxia
Intratumor hypoxia is a characteristic feature of solid tumors and hypoxic cells are proved to be resistant to ionizing radiation (33). Higher radiosensitivity of normoxic compared with hypoxic cells is most likely due to fixation of reactive oxygen species under normoxic conditions (34). Hypoxia has been constantly shown to be negatively associated with radiotherapy treatment response in head and neck cancers (35,36). Pathological hypoxic biomarkers have been studied in NPC biopsy samples; in particular, the transcription factor hypoxia inducible factor (HIF) 1-α and the genes up-regulated by HIF-1 such as carbonic anhydrase 9 (CA9) were studied as endogenous markers (37,38). Such intrinsic marker of hypoxia would have the advantage of being assessable on routine clinical biopsies. Besides, parameters of magnetic resonance perfusion-weighted imaging (MR-PWI) were found to be correlated with the expression of hypoxia-labelled markers, such as HIF-1α, vascular endothelial growth factor (VEGF), and microvessel density (MVD) in NPC, which is a promising new approach to predicting tumor hypoxia (39).
In addition to molecular profiles, hypoxia PET imaging has been gradually put into clinical use to detect tumor hypoxia, so as to selecting patients for radiation dose escalation or hypoxia-targeting therapy (40). In HNSCC, studies have suggested that boosting hypoxic tumor volume using baseline hypoxia imaging is feasible in principle, since 70–80% of the hypoxic volume is reproducible in test-retest experiments, while a small transient component was also seen in some patients (41-43). However, this test-retest experiment should be performed in NPC to confirm the stability of hypoxic volume in NPC tumor before its implementation in clinical practice. Moreover, if multiple images are obtained, it might be possible to increase the stability of hypoxic volume definition.
In NPC, using the hypoxia imaging agent fluorine-18 fluoromisonidazole (18F-FMISO) with positron emission tomography, tumor hypoxia was demonstrated in 100% of primary tumor and 58% of cervical lymph nodes metastases (44). Hypoxia detection using 18F-FMISO PET/CT and fluorine-18 fluoroazomycin arabinoside (18F-FAZA) PET/CT imaging have been found to have a prognostic and personalized radiotherapy potential in many cancers including NPC (45,46). A preliminary study showed that 18F-FMISO PET/CT-guided imaging hypoxia-targeted dose boost (an escalation of 20% of the prescription dose) was technically feasible by using volumetric-modulated arc therapy (VMAT) in NPC (45).
In addition to dose boost, the hypoxic cell radiosensitizer nimorazole has been successfully introduced to reduce the negative impact of hypoxia on the radiosensitivity of tumor cells in HNSCC (47,48). 18F-FAZA PET has shown some advantage in guiding personalized radiotherapy using nimorazole as radiosensitizer in rhabdomyosarcomas and esophagus adenocarcinoma in preclinical studies (46,49). However, the effectiveness and safety of nimorazole in NPC need to be tested in preclinical and clinical studies. Moreover, several hypoxia-activated prodrugs, such as evofosfamide (TH-302) and tirapazamine (TPZ), have been evaluated in preclinical studies in NPC to show therapeutic potential (50,51). However, there is still a long way to go before putting into clinical use.
Autophagy
Autophagy is another crucial mechanism of tumor cell death induced by radiation (52), and inhibition of autophagy enhances radiosensitivity of cancer cells in several cancer cell types (53) as well as in NPC (54). However, the mechanism remains unclear. Mo et al. demonstrated that inhibition of autophagy enhances the susceptibility of NPC cells to radiation by suppress recombination protein A (Rad51) expression (55). Rad51 is a key protein of homologous recombination that has been demonstrated to play a critical role in the repair of DNA DSBs induced by radiation (55). Therefore, Rad51 targeted therapy may be investigated as a potential novel agent for radiosensitization of NPC.
Contrarily, another study revealed that Annexin A6 (ANXA6) could promote autophagy by inhibiting the PI3K/AKT/mTOR pathway and it thus contributes to radioresistance of NPC, while ANXA6 siRNA suppressed cellular autophagy by activating the PI3K/AKT/mTOR pathway, ultimately leading to radiosensitization (56). Consequently, the combination of siANXA6 and CAL101 (an inhibitor of PI3K, p-AKT, and mTOR, concurrently) significantly reversed the above siANAX6-reduced autophagy. Preclinical study has also demonstrated that targeting the PI3K/mTOR pathway by dual PI3K/mTOR inhibitors (GSK2126458 and PKI-587) increased the radiosensitivity of NPC (57). GSK2126458 and PKI-587 are highly selective and potent small-molecule inhibitors which could effectively suppress both multiple class I PI3K isoforms and mTOR kinase activity (58,59). Both studies indicated that the combination of radiotherapy with PI3K/mTOR inhibitor, might be a promising therapeutic strategy for personalized radiotherapy of NPC.
Radiosensitizers
Radiosensitizers are intended to enhance cancer killing by ionizing radiation while having much less effect on normal tissues. Gemcitabine, a commonly used chemotherapy agent in NPC, can be used as a radiosensitizer and recent investigations have suggested that it markedly decreases oxygen consumption in tumor cells (60). Other than hypoxic cell radiosensitizer nimorazole, radiation sensitizers targeting the cellular mechanisms involved in the cell cycle, DNA repair or apoptosis pathways, have been studied in NPC. Sodium glycididazole (CMNA) is a commonly used radiosensitizer, when combined with radiotherapy in treating patients with locally advanced NPC, it could improve curative effects without increasing adverse reactions, and significantly increase survival rates of the patients (61). Preclinical study revealed that CMNA enhance the radiosensitivity of the NPC cells via enhancing DNA damage and promoting cell apoptosis (62). Besides, a bisbenzylisoquinoline alkaloid which is isolated from the roof of the Chinese herb Stephania tetrandra, termed as tetrandrine, is found to be effective in enhancing the radiosensitivity of NPC cancer cells and the underlying mechanism could be associated with abrogation of radiation-induced G2/M arrest via activation of the CDC25C/CDK1/Cyclin B1 pathway (63,64). However, these agents are not routinely used in clinical practice of NPC. In the future, radiosensitizers targeting the mechanisms of radioresistance should be further investigated in preclinical and clinical studies.
Future perspectives and challenges
The experimental evidences to date suggest that mechanisms such as DNA damage repair, tumor hypoxia and autophagy, play sizable roles in radioresistance of NPC, targeting these mechanisms is one of the most promising aspects in personalized radiotherapy. In order to guide the most effective way to develop radiosensitizers, it will be important to investigate in depth: (I) genes, signaling pathways, miRNAs or lncRNAs to regulate radioresistance; (II) blocking approaches for tumor cells to endow CSC phenotype or sensitize CSCs to iron irradiation; (III) how EBV interact with cancer cells or adjacent cells to promote NPC radioresistance. Most importantly, biomarker-driven stratification for use of radiosensitizer and radiation dose escalation or de-escalation are worthy of exploration in the future studies. On the other hand, pilot studies have demonstrated that hypoxia imaging has the potential to guide personalized hypoxic dose painting radiotherapy. In the future, these personalized strategies should be verified in well-designed preclinical studies and prospective clinical trials for parallel investigation of biological mechanisms and patient outcomes.
The arrival of new era of immunotherapy have provided unprecedented opportunities for personalized radiotherapy by optimization of strategies combining radiotherapy with immunotherapy, to improve both local and systemic tumor control. It is now recognized that, in parallel with killing tumor cells, radiotherapy can enhance the efficacy of immunotherapy agents by releasing vaccine in situ, improving antigen presentation, removing the inhibitory immune microenvironment, and increasing programmed death-ligand 1 (PD-L1) expression of tumor cells (65-68). Promising results have been reported with radiotherapy and immunotherapy in pre-clinical and pilot clinical studies (69,70). Widely clinical translation requires deep understanding of the complex interaction between radiotherapy and immune cells, cancer cells and particular immunotherapy agents; as well as investigation of the sequencing of the immunotherapy agent relative to radiotherapy, and the site and route of delivery of the immunotherapy agents.
A major challenge emerging for clinical research on personalized radiotherapy is that patient cohorts when precisely stratified by biomarkers get smaller. Hence, traditional phase III randomized control trials, which require large numbers of patients with the same kind of cancer, is no longer the best trial design. This warrants adaptation and further development of trial designs to assemble solid evidence for personalized treatment options. In the future, the important issue is optimization of the trial design and quality of preclinical studies in order to achieve positive clinical results.
Conclusions
In NPC, personalized radiotherapy is actually an under-researched area. Based on current preclinical experiments and pilot clinical studies, potential personalized strategies will include developing radiosensitizers targeting the mechanisms of radioresistance of NPC, biomarker-driven stratification for use of the radiosensitizers and radiation dose escalation or de-escalation, and personalized hypoxic dose painting radiotherapy guided by hypoxia imaging. In the future, these personalized strategies should be verified in well-designed preclinical studies and prospective clinical trials for parallel investigation of biological mechanisms and patient outcomes.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Wai-Tong Ng, Sophie (Shao Hui) Huang, Hai-Qiang Mai) for the special series “Precision Radiotherapy in Nasopharyngeal Carcinoma” published in Annals of Nasopharynx Cancer. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/anpc-20-20). The series “Precision Radiotherapy in Nasopharyngeal Carcinoma” was commissioned by the editorial office without any funding or sponsorship. YPC serves as an unpaid editorial board member of Annals of Nasopharynx Cancer from Aug 2019 to Aug 2021. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kam MK, Chau RM, Suen J, et al. Intensity-modulated radiotherapy in nasopharyngeal carcinoma: dosimetric advantage over conventional plans and feasibility of dose escalation. Int J Radiat Oncol Biol Phys 2003;56:145-57. [Crossref] [PubMed]
- Liu J, Lyman KM, Ding Z, et al. Assessment of the therapeutic accuracy of cone beam computed tomography-guided nasopharyngeal carcinoma radiotherapy. Oncol Lett 2019;18:1071-80. [Crossref] [PubMed]
- Zhang MX, Li J, Shen GP, et al. Intensity-modulated radiotherapy prolongs the survival of patients with nasopharyngeal carcinoma compared with conventional two-dimensional radiotherapy: A 10-year experience with a large cohort and long follow-up. Eur J Cancer 2015;51:2587-95. [Crossref] [PubMed]
- Lee AW, Tung SY, Chan AT, et al. Preliminary results of a randomized study (NPC-9902 Trial) on therapeutic gain by concurrent chemotherapy and/or accelerated fractionation for locally advanced nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 2006;66:142-51. [Crossref] [PubMed]
- Zeng L, Tian YM, Sun XM, et al. Late toxicities after intensity-modulated radiotherapy for nasopharyngeal carcinoma: patient and treatment-related risk factors. Br J Cancer 2014;110:49-54. [Crossref] [PubMed]
- Roper N, Stensland KD, Hendricks R, et al. The landscape of precision cancer medicine clinical trials in the United States. Cancer Treat Rev 2015;41:385-90. [Crossref] [PubMed]
- Fountzilas E, Tsimberidou AM. Overview of precision oncology trials: challenges and opportunities. Expert Rev Clin Pharmacol 2018;11:797-804. [Crossref] [PubMed]
- Chen AM, Felix C, Wang PC, et al. Reduced-dose radiotherapy for human papillomavirus-associated squamous-cell carcinoma of the oropharynx: a single-arm, phase 2 study. Lancet Oncol 2017;18:803-11. [Crossref] [PubMed]
- Marur S, Li S, Cmelak AJ, et al. E1308: Phase II Trial of Induction Chemotherapy Followed by Reduced-Dose Radiation and Weekly Cetuximab in Patients With HPV-Associated Resectable Squamous Cell Carcinoma of the Oropharynx- ECOG-ACRIN Cancer Research Group. J Clin Oncol 2017;35:490-7. [Crossref] [PubMed]
- Scott JG, Berglund A, Schell MJ, et al. A genome-based model for adjusting radiotherapy dose (GARD): a retrospective, cohort-based study. Lancet Oncol 2017;18:202-11. [Crossref] [PubMed]
- Wang X, Wang Y, Jiang S, et al. Safety and Effectiveness of De-escalated Radiation Dose in T1-3 Nasopharyngeal Carcinoma: A Propensity Matched Analysis. J Cancer 2019;10:5057-64. [Crossref] [PubMed]
- Chen FP, Lin L, Qi ZY, et al. Pretreatment Nomograms for Local and Regional Recurrence after Radical Radiation Therapy for Primary Nasopharyngeal Carcinoma. J Cancer 2017;8:2595-603. [Crossref] [PubMed]
- Zhang LL, Huang MY, Li Y, et al. Pretreatment MRI radiomics analysis allows for reliable prediction of local recurrence in non-metastatic T4 nasopharyngeal carcinoma. EBioMedicine 2019;42:270-80. [Crossref] [PubMed]
- Liu B, Tan Z, Jiang Y, et al. Correlation between the expression of miR150 and FOXO4 and the local recurrence and metastasis of nasopharyngeal carcinoma after intensive radiotherapy. J BUON 2018;23:1671-8. [PubMed]
- Liu F, Xi XP, Wang H, et al. PET/CT-guided dose-painting versus CT-based intensity modulated radiation therapy in locoregional advanced nasopharyngeal carcinoma. Radiat Oncol 2017;12:15. [Crossref] [PubMed]
- Dionisi F, Croci S, Giacomelli I, et al. Clinical results of proton therapy reirradiation for recurrent nasopharyngeal carcinoma. Acta Oncol 2019;58:1238-45. [Crossref] [PubMed]
- Hu J, Bao C, Gao J, et al. Salvage treatment using carbon ion radiation in patients with locoregionally recurrent nasopharyngeal carcinoma: Initial results. Cancer 2018;124:2427-37. [Crossref] [PubMed]
- Lomax ME, Folkes LK, O'Neill P. Biological consequences of radiation-induced DNA damage: relevance to radiotherapy. Clin Oncol (R Coll Radiol) 2013;25:578-85. [Crossref] [PubMed]
- Qu C, Zhao Y, Feng G, et al. RPA3 is a potential marker of prognosis and radioresistance for nasopharyngeal carcinoma. J Cell Mol Med 2017;21:2872-83. [Crossref] [PubMed]
- Leszczynska KB, Foskolou IP, Abraham AG, et al. Hypoxia-induced p53 modulates both apoptosis and radiosensitivity via AKT. J Clin Invest 2015;125:2385-98. [Crossref] [PubMed]
- Kimple RJ, Smith MA, Blitzer GC, et al. Enhanced radiation sensitivity in HPV-positive head and neck cancer. Cancer Res 2013;73:4791-800. [Crossref] [PubMed]
- Huang S, Benavente S, Armstrong EA, et al. p53 modulates acquired resistance to EGFR inhibitors and radiation. Cancer Res 2011;71:7071-9. [Crossref] [PubMed]
- Lu J, Tang M, Li H, et al. EBV-LMP1 suppresses the DNA damage response through DNA-PK/AMPK signaling to promote radioresistance in nasopharyngeal carcinoma. Cancer Lett 2016;380:191-200. [Crossref] [PubMed]
- Di M, Wang M, Miao J, et al. CHAF1B induces radioresistance by promoting DNA damage repair in nasopharyngeal carcinoma. Biomed Pharmacother 2020;123:109748 [Crossref] [PubMed]
- Blanpain C, Mohrin M, Sotiropoulou PA, et al. DNA-damage response in tissue-specific and cancer stem cells. Cell Stem Cell 2011;8:16-29. [Crossref] [PubMed]
- Wang WJ, Wu SP, Liu JB, et al. MYC regulation of CHK1 and CHK2 promotes radioresistance in a stem cell-like population of nasopharyngeal carcinoma cells. Cancer Res 2013;73:1219-31. [Crossref] [PubMed]
- Lei F, Lei T, Huang Y, et al. Radio-Susceptibility of Nasopharyngeal Carcinoma: Focus on Epstein-Barr virus, MicroRNAs, Long non-coding RNAs and Circular RNAs. Curr Mol Pharmacol 2020;13:192-205. [Crossref] [PubMed]
- Hu Z, Zhou S, Luo H, et al. miRNA-17 promotes nasopharyngeal carcinoma radioresistance by targeting PTEN/AKT. Int J Clin Exp Pathol 2019;12:229-40. [PubMed]
- Wu W, Chen X, Yu S, et al. microRNA-222 promotes tumor growth and confers radioresistance in nasopharyngeal carcinoma by targeting PTEN. Mol Med Rep 2018;17:1305-10. [PubMed]
- Qu JQ, Yi HM, Ye X, et al. MiRNA-203 Reduces Nasopharyngeal Carcinoma Radioresistance by Targeting IL8/AKT Signaling. Mol Cancer Ther 2015;14:2653-64. [Crossref] [PubMed]
- He Y, Jing Y, Wei F, et al. Long non-coding RNA PVT1 predicts poor prognosis and induces radioresistance by regulating DNA repair and cell apoptosis in nasopharyngeal carcinoma. Cell Death Dis 2018;9:235. [Crossref] [PubMed]
- Hu X, Jiang H, Jiang X. Downregulation of lncRNA ANRIL inhibits proliferation, induces apoptosis, and enhances radiosensitivity in nasopharyngeal carcinoma cells through regulating miR-125a. Cancer Biol Ther 2017;18:331-8. [Crossref] [PubMed]
- Thomlinson RH, Gray LH. The histological structure of some human lung cancers and the possible implications for radiotherapy. Br J Cancer 1955;9:539-49. [Crossref] [PubMed]
- Gray LH, Conger AD, Ebert M, et al. The concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. Br J Radiol 1953;26:638-48. [Crossref] [PubMed]
- Brizel DM, Dodge RK, Clough RW, et al. Oxygenation of head and neck cancer: changes during radiotherapy and impact on treatment outcome. Radiother Oncol 1999;53:113-7. [Crossref] [PubMed]
- Nordsmark M, Overgaard J. Tumor hypoxia is independent of hemoglobin and prognostic for loco-regional tumor control after primary radiotherapy in advanced head and neck cancer. Acta Oncol 2004;43:396-403. [Crossref] [PubMed]
- Hui EP, Chan AT, Pezzella F, et al. Coexpression of hypoxia-inducible factors 1alpha and 2alpha, carbonic anhydrase IX, and vascular endothelial growth factor in nasopharyngeal carcinoma and relationship to survival. Clin Cancer Res 2002;8:2595-604. [PubMed]
- Sung FL, Hui EP, Tao Q, et al. Genome-wide expression analysis using microarray identified complex signaling pathways modulated by hypoxia in nasopharyngeal carcinoma. Cancer Lett 2007;253:74-88. [Crossref] [PubMed]
- Hu Y. Correlation of quantitative parameters of magnetic resonance perfusion-weighted imaging with vascular endothelial growth factor, microvessel density and hypoxia-inducible factor-1α in nasopharyngeal carcinoma: Evaluation on radiosensitivity study. Clin Otolaryngol 2018;43:425-33. [Crossref] [PubMed]
- Peeters SG, Zegers CM, Yaromina A, et al. Current preclinical and clinical applications of hypoxia PET imaging using 2-nitroimidazoles. Q J Nucl Med Mol Imaging 2015;59:39-57. [PubMed]
- Nehmeh SA, Lee NY, Schröder H, et al. Reproducibility of intratumor distribution of (18)F-fluoromisonidazole in head and neck cancer. Int J Radiat Oncol Biol Phys 2008;70:235-42. [Crossref] [PubMed]
- Bittner MI, Wiedenmann N, Bucher S, et al. Exploratory geographical analysis of hypoxic subvolumes using 18F-MISO-PET imaging in patients with head and neck cancer in the course of primary chemoradiotherapy. Radiother Oncol 2013;108:511-6. [Crossref] [PubMed]
- Bittner MI, Grosu AL. Hypoxia in head and neck tumors: characteristics and development during therapy. Front Oncol 2013;3:223. [Crossref] [PubMed]
- Yeh SH, Liu RS, Wu LC, et al. Fluorine-18 fluoromisonidazole tumour to muscle retention ratio for the detection of hypoxia in nasopharyngeal carcinoma. Eur J Nucl Med 1996;23:1378-83. [Crossref] [PubMed]
- Qiu J, Lv B, Fu M, et al. 18 F-Fluoromisonidazole positron emission tomography/CT-guided volumetric-modulated arc therapy-based dose escalation for hypoxic subvolume in nasopharyngeal carcinomas: A feasibility study. Head Neck 2017;39:2519-27. [Crossref] [PubMed]
- Tran LB, Bol A, Labar D, et al. Predictive value of (18)F-FAZA PET imaging for guiding the association of radiotherapy with nimorazole: a preclinical study. Radiother Oncol 2015;114:189-94. [Crossref] [PubMed]
- Meißner R, Kočišek J, Feketeová L, et al. Low-energy electrons transform the nimorazole molecule into a radiosensitiser. Nat Commun 2019;10:2388. [Crossref] [PubMed]
- Metwally MA, Frederiksen KD, Overgaard J. Compliance and toxicity of the hypoxic radiosensitizer nimorazole in the treatment of patients with head and neck squamous cell carcinoma (HNSCC). Acta Oncol 2014;53:654-61. [Crossref] [PubMed]
- Melsens E, De Vlieghere E, Descamps B, et al. Hypoxia imaging with 18F-FAZA PET/CT predicts radiotherapy response in esophageal adenocarcinoma xenografts. Radiat Oncol 2018;13:39. [Crossref] [PubMed]
- Huang Y, Tian Y, Zhao Y, et al. Efficacy of the hypoxia-activated prodrug evofosfamide (TH-302) in nasopharyngeal carcinoma in vitro and in vivo. Cancer Commun (Lond) 2018;38:15. [Crossref] [PubMed]
- Hong B, Lui VW, Hui EP, et al. Hypoxia-targeting by tirapazamine (TPZ) induces preferential growth inhibition of nasopharyngeal carcinoma cells with Chk1/2 activation. Invest New Drugs 2011;29:401-10. [Crossref] [PubMed]
- Zois CE, Koukourakis MI. Radiation-induced autophagy in normal and cancer cells: towards novel cytoprotection and radio-sensitization policies?. Autophagy 2009;5:442-50. [Crossref] [PubMed]
- Apel A, Herr I, Schwarz H, et al. Blocked autophagy sensitizes resistant carcinoma cells to radiation therapy. Cancer Res 2008;68:1485-94. [Crossref] [PubMed]
- Wang Y, Yin W, Zhu X. Blocked autophagy enhances radiosensitivity of nasopharyngeal carcinoma cell line CNE-2 in vitro. Acta Otolaryngol 2014;134:105-10. [Crossref] [PubMed]
- Mo N, Lu YK, Xie WM, et al. Inhibition of autophagy enhances the radiosensitivity of nasopharyngeal carcinoma by reducing Rad51 expression. Oncol Rep 2014;32:1905-12. [Crossref] [PubMed]
- Chen Q, Zheng W, Zhu L, et al. ANXA6 Contributes to Radioresistance by Promoting Autophagy via Inhibiting the PI3K/AKT/mTOR Signaling Pathway in Nasopharyngeal Carcinoma. Front Cell Dev Biol 2020;8:232. [Crossref] [PubMed]
- Liu T, Sun Q, Li Q, et al. Dual PI3K/mTOR inhibitors, GSK2126458 and PKI-587, suppress tumor progression and increase radiosensitivity in nasopharyngeal carcinoma. Mol Cancer Ther 2015;14:429-39. [Crossref] [PubMed]
- Leung E, Kim JE, Rewcastle GW, et al. Comparison of the effects of the PI3K/mTOR inhibitors NVP-BEZ235 and GSK2126458 on tamoxifen-resistant breast cancer cells. Cancer Biol Ther 2011;11:938-46. [Crossref] [PubMed]
- Mallon R, Feldberg LR, Lucas J, et al. Antitumor efficacy of PKI-587, a highly potent dual PI3K/mTOR kinase inhibitor. Clin Cancer Res 2011;17:3193-203. [Crossref] [PubMed]
- Grimes DR, Kannan P, McIntyre A, et al. The Role of Oxygen in Avascular Tumor Growth. PLoS One 2016;11:e0153692 [Crossref] [PubMed]
- Liu MZ, He LR, Lu TX, et al. Zhonghua Zhong Liu Za Zhi 2006;28:932-7. [Effect of hypoxic radiosensitizer sodium glycididazole on long-term result of radiotherapy for nasopharyngeal carcinoma]. [PubMed]
- Min X, Huang F, Huang H, et al. The Radiosensitization of Sodium Glycididazole on Nasopharyngeal Carcinoma Cells via Enhancing DNA Damage and Promoting Apoptosis. J Cancer 2019;10:305-12. [Crossref] [PubMed]
- Wang J, Chang L, Lai X, et al. Tetrandrine enhances radiosensitivity through the CDC25C/CDK1/cyclin B1 pathway in nasopharyngeal carcinoma cells. Cell Cycle 2018;17:671-80. [Crossref] [PubMed]
- Chen YJ. Potential role of tetrandrine in cancer therapy. Acta Pharmacol Sin 2002;23:1102-6. [PubMed]
- Yokouchi H, Yamazaki K, Chamoto K, et al. Anti-OX40 monoclonal antibody therapy in combination with radiotherapy results in therapeutic antitumor immunity to murine lung cancer. Cancer Sci 2008;99:361-7. [Crossref] [PubMed]
- Barker HE, Paget JT, Khan AA, et al. The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nat Rev Cancer 2015;15:409-25. [Crossref] [PubMed]
- Reynders K, Illidge T, Siva S, et al. The abscopal effect of local radiotherapy: using immunotherapy to make a rare event clinically relevant. Cancer Treat Rev 2015;41:503-10. [Crossref] [PubMed]
- Dovedi SJ, Adlard AL, Lipowska-Bhalla G, et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res 2014;74:5458-68. [Crossref] [PubMed]
- Rodriguez-Ruiz ME, Rodriguez I, Garasa S, et al. Abscopal Effects of Radiotherapy Are Enhanced by Combined Immunostimulatory mAbs and Are Dependent on CD8 T Cells and Crosspriming. Cancer Res 2016;76:5994-6005. [Crossref] [PubMed]
- Theelen WSME, Peulen HMU, Lalezari F, et al. Effect of Pembrolizumab After Stereotactic Body Radiotherapy vs Pembrolizumab Alone on Tumor Response in Patients With Advanced Non-Small Cell Lung Cancer: Results of the PEMBRO-RT Phase 2 Randomized Clinical Trial. JAMA Oncol 2019;5:1276-82. [Crossref] [PubMed]
Cite this article as: Lin L, Chen YP, Sun Y. Biological opportunities for personalized radiotherapy of nasopharyngeal carcinoma. Ann Nasopharynx Cancer 2021;5:2.


